Question: 6. In the class, we have gone through the example of polyenes in the context of the 1. D PIB model. Now, let's work on
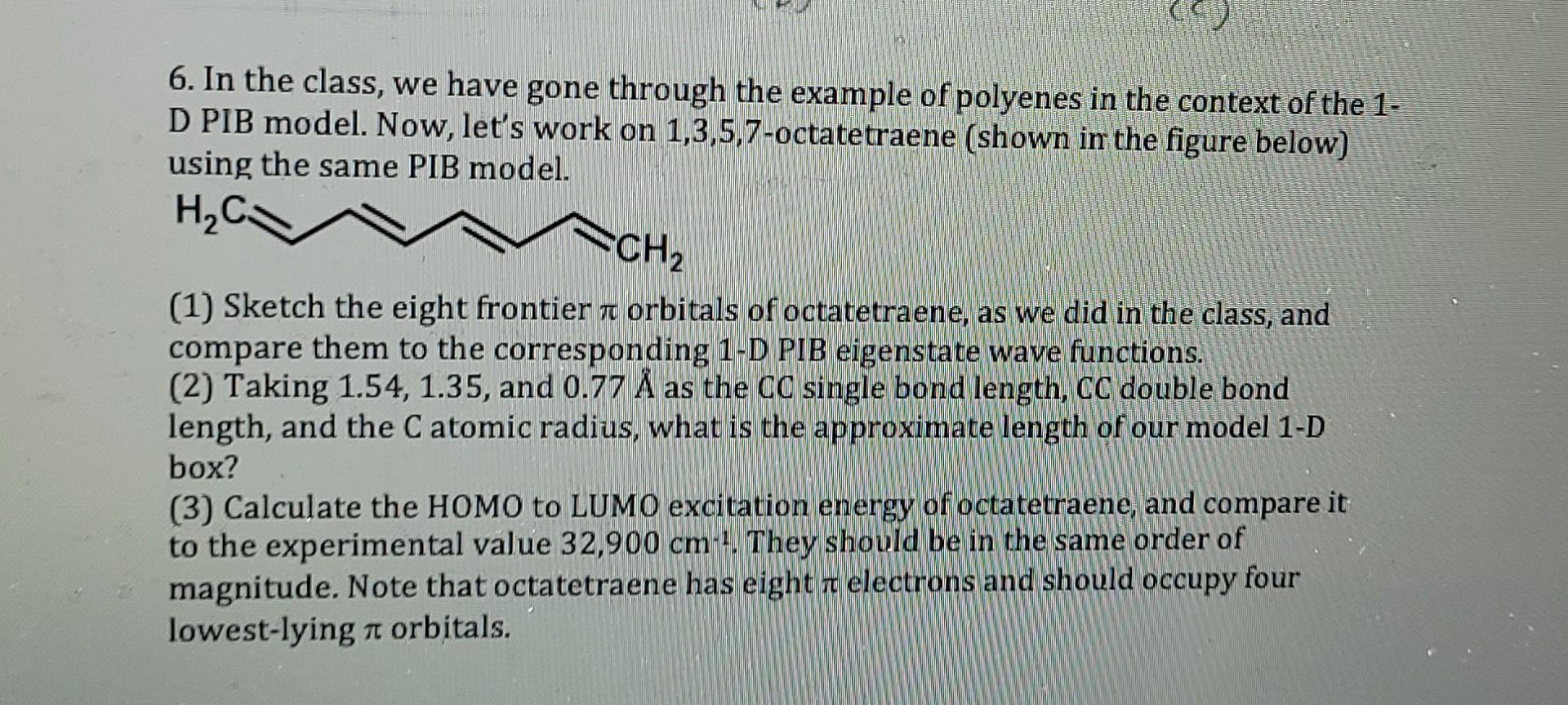
6. In the class, we have gone through the example of polyenes in the context of the 1. D PIB model. Now, let's work on 1,3,5,7-octatetraene (shown in the figure below) using the same PIB model. HC. CH2 TO (1) Sketch the eight frontier a orbitals of octatetraene, as we did in the class, and compare them to the corresponding 1-D PIB eigenstate wave functions. (2) Taking 1.54, 1.35, and 0.77 as the CC single bond length, CC double bond length, and the C atomic radius, what is the approximate length of our model 1-D box? (3) Calculate the HOMO to LUMO excitation energy of octatetraene, and compare it to the experimental value 32,900 cm 1. They should be in the same order of magnitude. Note that octatetraene has eight r electrons and should occupy four lowest-lying n orbitals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


