Question: 6) In the data table, there are no anions listed. We know that we cannot have a cation present without an equal opposite charged anion(s)
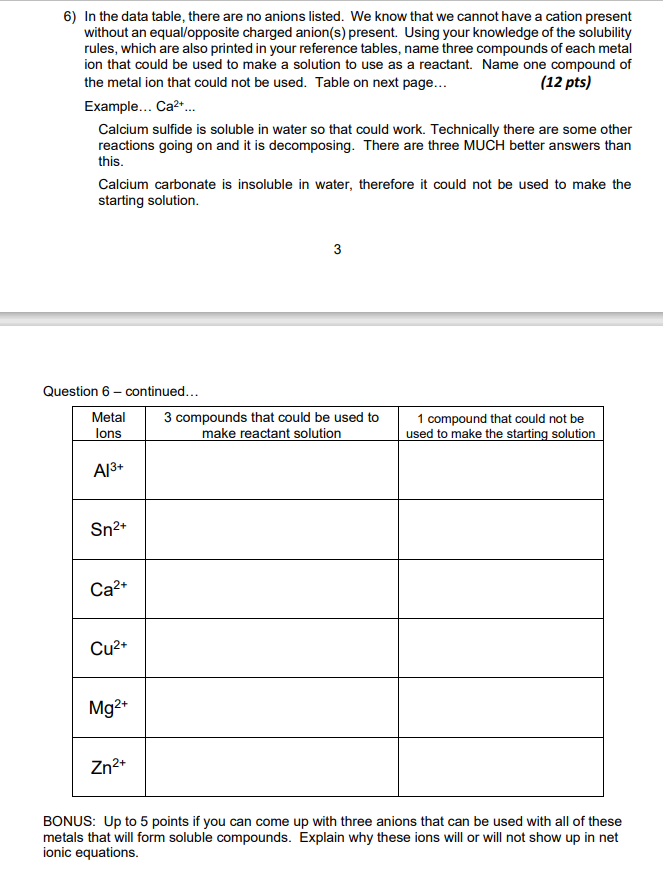
6) In the data table, there are no anions listed. We know that we cannot have a cation present without an equal opposite charged anion(s) present. Using your knowledge of the solubility rules, which are also printed in your reference tables, name three compounds of each metal ion that could be used to make a solution to use as a reactant. Name one compound of the metal ion that could not be used. Table on next page... (12 pts) Example... Ca2+... Calcium sulfide is soluble in water so that could work. Technically there are some other reactions going on and it is decomposing. There are three MUCH better answers than this. Calcium carbonate is insoluble in water, therefore it could not be used to make the starting solution. 3 Question 6 - continued... Metal 3 compounds that could be used to lons make reactant solution 1 compound that could not be used to make the starting solution Al3+ Sn2+ Ca2+ Cu2+ Mg2+ Zn2+ BONUS: Up to 5 points if you can come up with three anions that can be used with all of these metals that will form soluble compounds. Explain why these ions will or will not show up in net ionic equations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


