Question: 6 points KNOWLEDGE: Answer the questions below by hand. All work must be properly communicated for full marks. 1. A 0.554 L canister of compressed

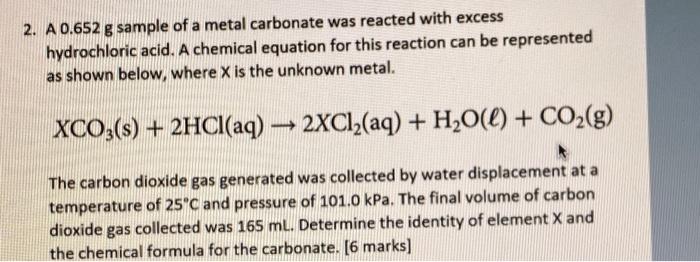
6 points KNOWLEDGE: Answer the questions below by hand. All work must be properly communicated for full marks. 1. A 0.554 L canister of compressed gas has a pressure of 1502 Torr at standard ambient temperature. a. Assuming that the canister is rigid and can withstand pressures up to 2460 Torr, can the container be safely heated to 112 C? [3 marks) b. If the canister were instead perfectly flexible such that it always maintains a constant pressure, what volume would it attain at 112 C? [3 marks) 2. A 0.652 g sample of a metal carbonate was reacted with excess hydrochloric acid. A chemical equation for this reaction can be represented as shown below, where X is the unknown metal. XCO3(s) + 2HCl(aq) 2XC12(aq) + H2O(l) + CO2(g) The carbon dioxide gas generated was collected by water displacement at a temperature of 25C and pressure of 101.0 kPa. The final volume of carbon dioxide gas collected was 165 ml. Determine the identity of element X and the chemical formula for the carbonate. [6 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


