Question: 6. The following table shows the experimental surface tension data for an aqueous solution of nonylphenyl ethoxylate NPE15 at 20C (the subscript 15 indicates that
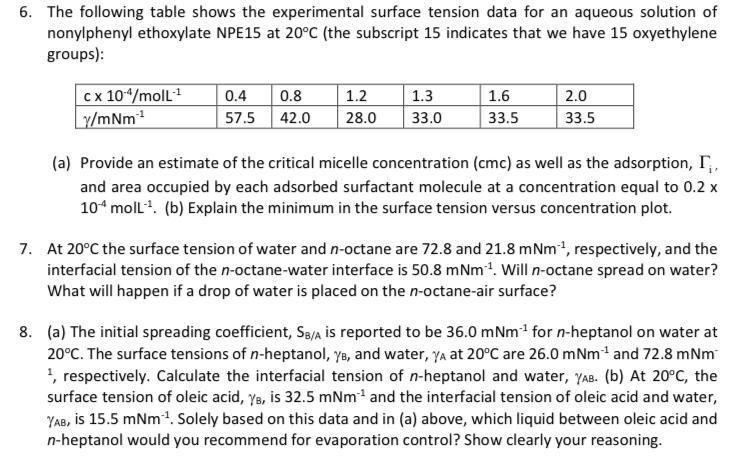
6. The following table shows the experimental surface tension data for an aqueous solution of nonylphenyl ethoxylate NPE15 at 20C (the subscript 15 indicates that we have 15 oxyethylene groups): cx 10"/moll y/mNm? 0.4 0.8 57.5 42.0 1.2 28.0 1.3 33.0 1.6 33.5 2.0 33.5 (a) Provide an estimate of the critical micelle concentration (cmc) as well as the adsorption, T, and area occupied by each adsorbed surfactant molecule at a concentration equal to 0.2 x 10moll? (b) Explain the minimum in the surface tension versus concentration plot. 7. At 20C the surface tension of water and n-octane are 72.8 and 21.8 mNm-, respectively, and the interfacial tension of the n-octane-water interface is 50.8 mNm 1. Will n-octane spread on water? What will happen if a drop of water is placed on the n-octane-air surface? 8. (a) The initial spreading coefficient, SB/A is reported to be 36.0 m Nm' for n-heptanol on water at 20C. The surface tensions of n-heptanol, yo, and water, ya at 20C are 26.0 mNm- and 72.8 mNm , respectively. Calculate the interfacial tension of n-heptanol and water, YAB. (b) At 20C, the surface tension of oleic acid, ye, is 32.5 mNm and the interfacial tension of oleic acid and water, YAB, is 15.5 mNm + Solely based on this data and in (a) above, which liquid between oleic acid and n-heptanol would you recommend for evaporation control? Show clearly your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


