Question: 6. Using the spectral information below, please answer the following questions about an unknown compound with the molecular formula C10H10O2. (22 points) 'H NMR (
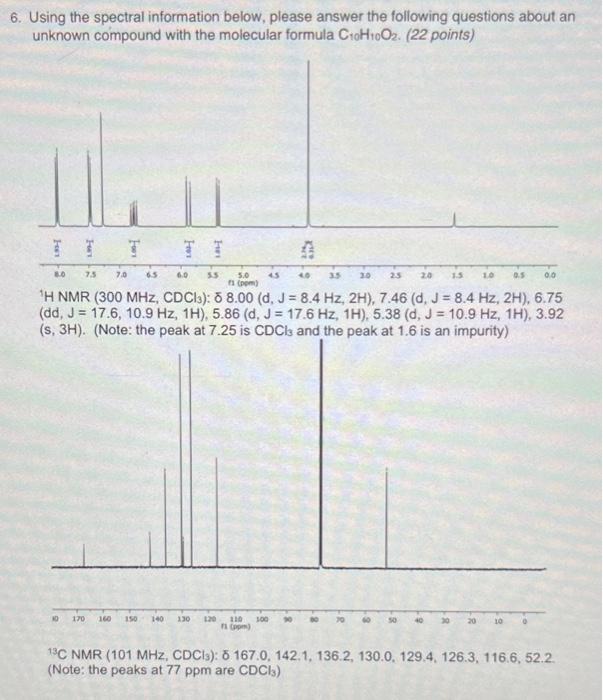

6. Using the spectral information below, please answer the following questions about an unknown compound with the molecular formula C10H10O2. (22 points) 'H NMR ( 300MHz,CDCl3):8.00(d,J=8.4Hz,2H),7.46(d,J=8.4Hz,2H),6.75 (dd, J=17.6,10.9Hz,1H),5.86(d,J=17.6Hz,1H),5.38(d,J=10.9Hz,1H),3.92 (s, 3H). (Note: the peak at 7.25 is CDCls and the peak at 1.6 is an impurity) (Note: the peaks at 77ppm are CDCl3 ) A) Calculate the degree of unsaturation for this molecule. (2 points) B) Propose functional groups that correspond with the following portions of structural data. For yet unknown bond connections (bonds that extend outside the proposed group, use squiggles over bonds). Example: Ethyl group with coupling constant relationship i. ' H NMR signals at 8.00(d,J=8.4Hz,2H),7.46(d,J=8.4Hz,2H) and 13C NMR signals at 142.1,130.0,129.4,126.3. (4 points) ii. 1H NMR signals at 6.75 (dd, J=17.6,10.9Hz,1H),5.86(d,J=17.6Hz, 1H),5.38(d,J=10.9Hz,1H) and 13C NMR signals at 136.2,116.6. Please indicate the coupling constants between protons and any stereochemical relationships. ( 5 points) iii. 13C NMR signal at 167.0. (3 points) iv. ' 1H NMR signal at 3.92(s,3H) and 13C NMR signal at 52.2. (3 points) C) Propose a structure for this molecule consistent with the data provided. Be sure to account for all degrees of unsaturation! (5 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


