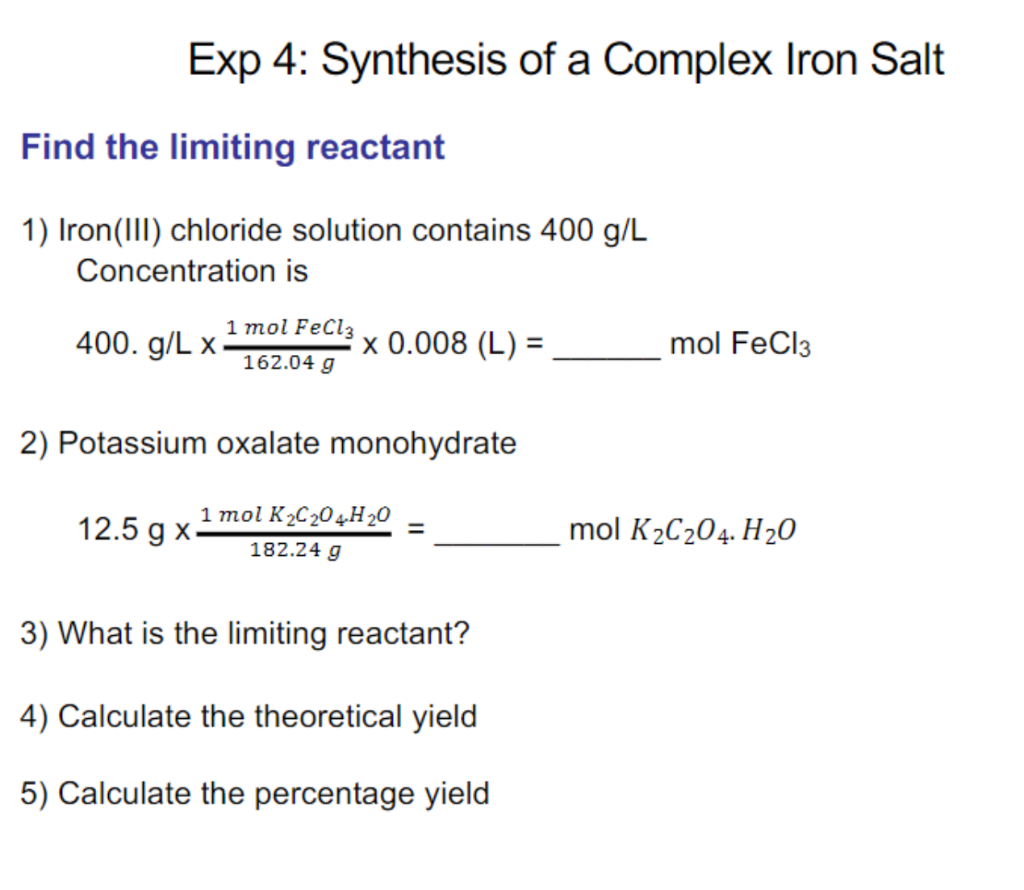
Question: 6) Which safety precautions do you need to take into consideration? 400.g/L162.04g1molFeCl30.008(L)=molFeCl3 2) Potassium oxalate monohydrate 12.5g182.24g1molK2C2O4H2O=molK2C2O4H2O 3) What is the limiting reactant? 4) Calculate
 6) Which safety precautions do you need to take into consideration?
6) Which safety precautions do you need to take into consideration?
400.g/L162.04g1molFeCl30.008(L)=molFeCl3 2) Potassium oxalate monohydrate 12.5g182.24g1molK2C2O4H2O=molK2C2O4H2O 3) What is the limiting reactant? 4) Calculate the theoretical yield 5) Calculate the percentage yield
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


