Question: 6:06 PM Sat Nov 18 . . . @ 78% calstatela.instructure.com - Privat Lic DateT adowater D Frage 2 5 Pkte. 2. How many grams
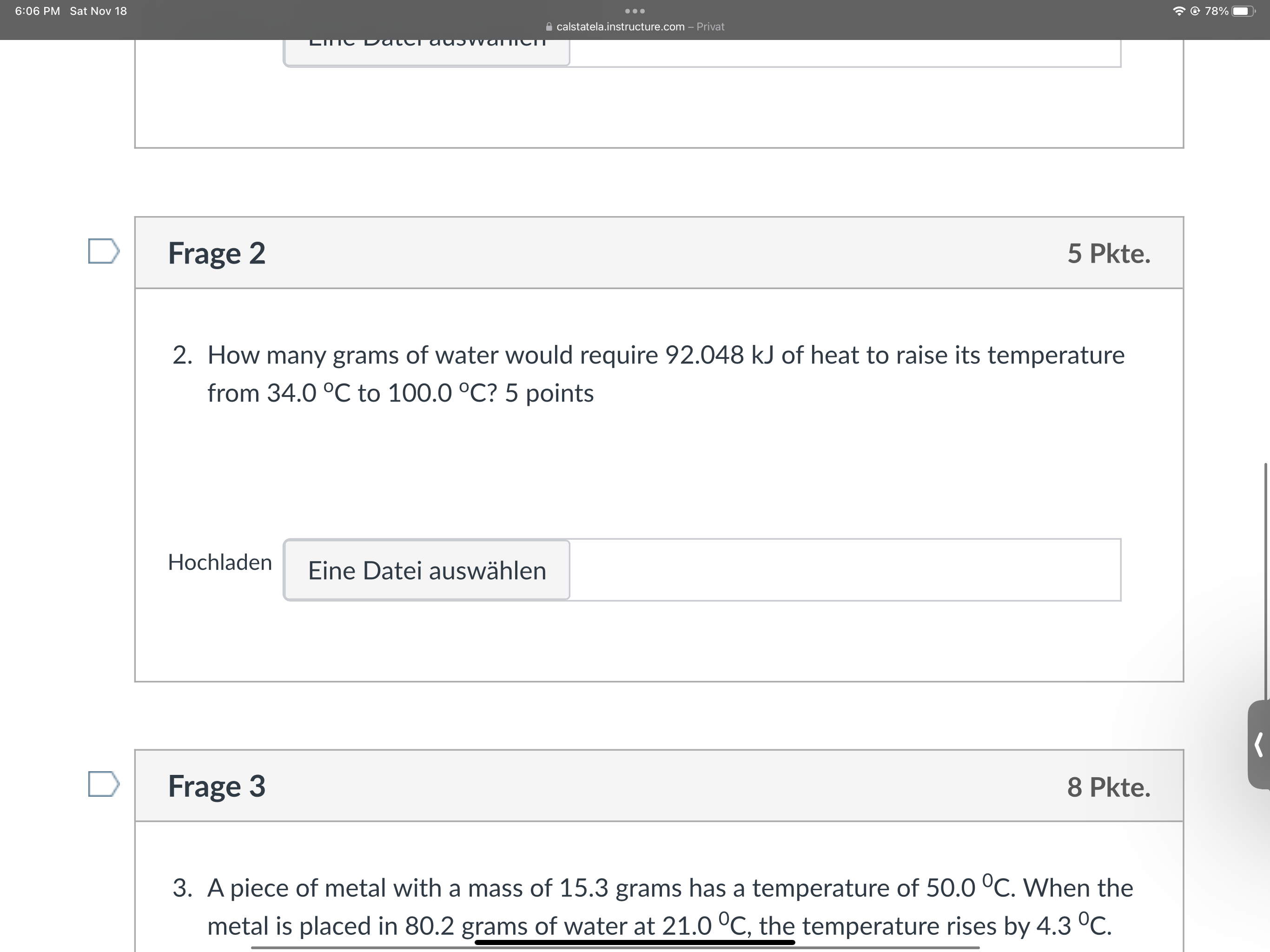
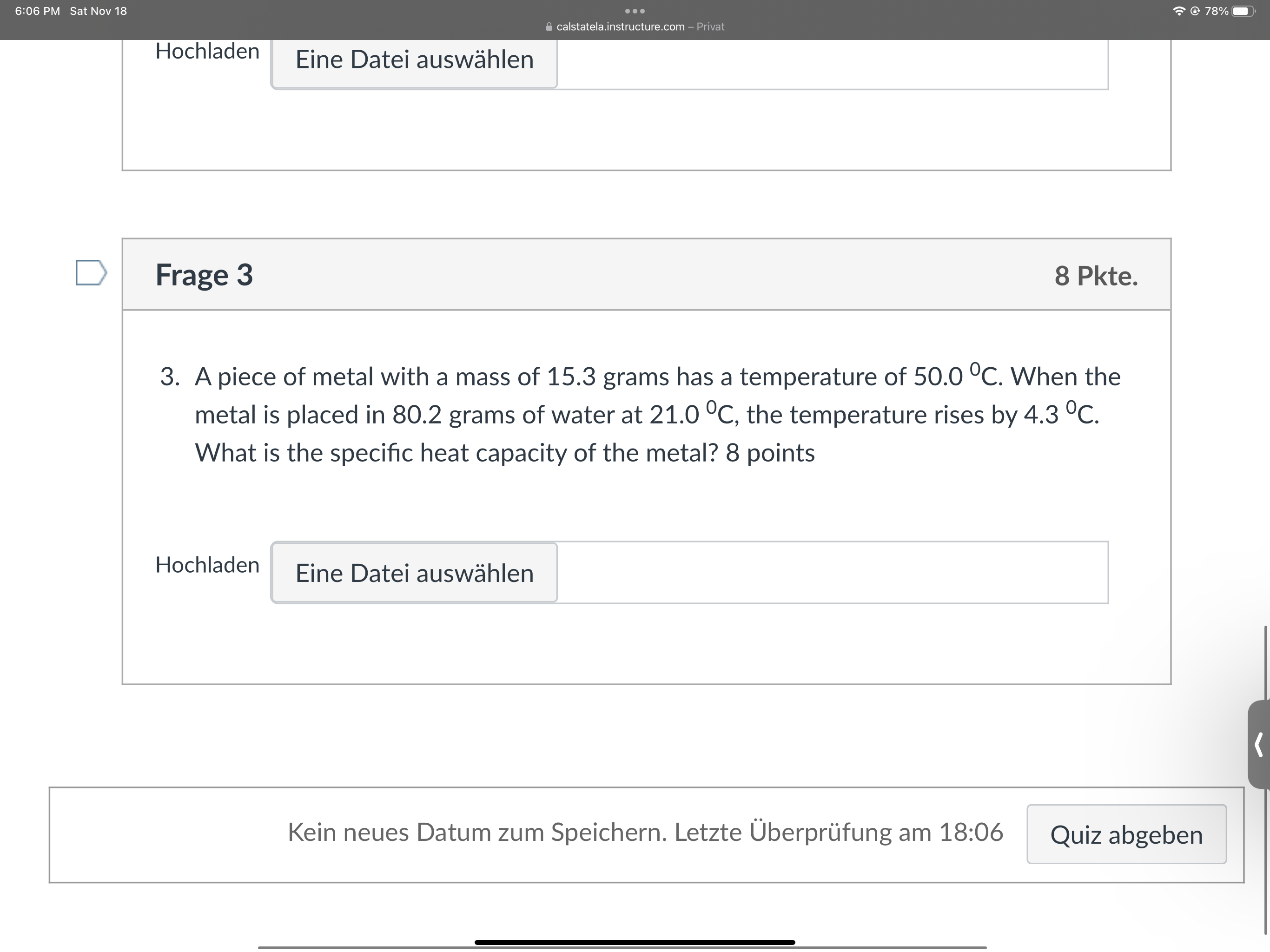
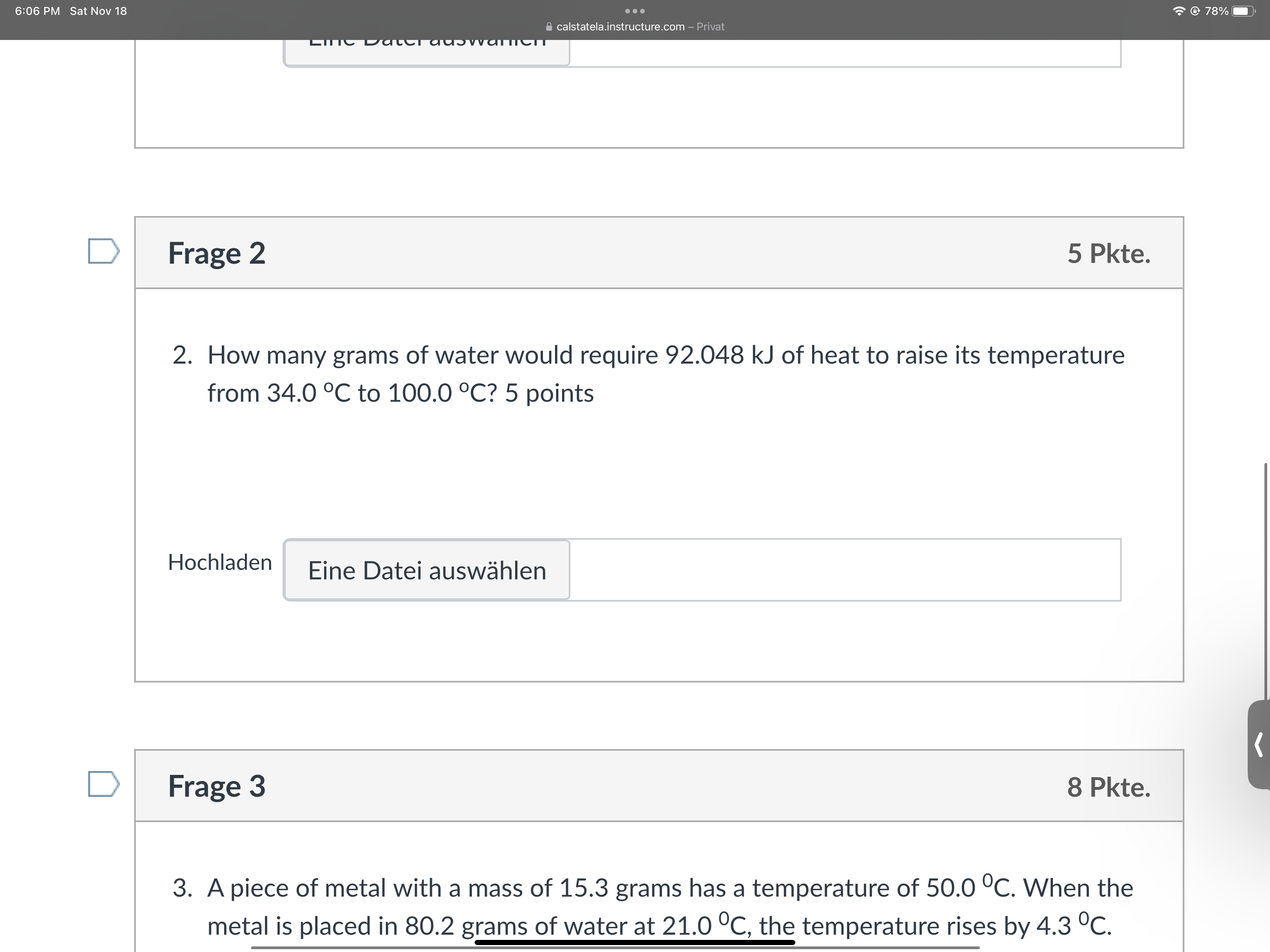
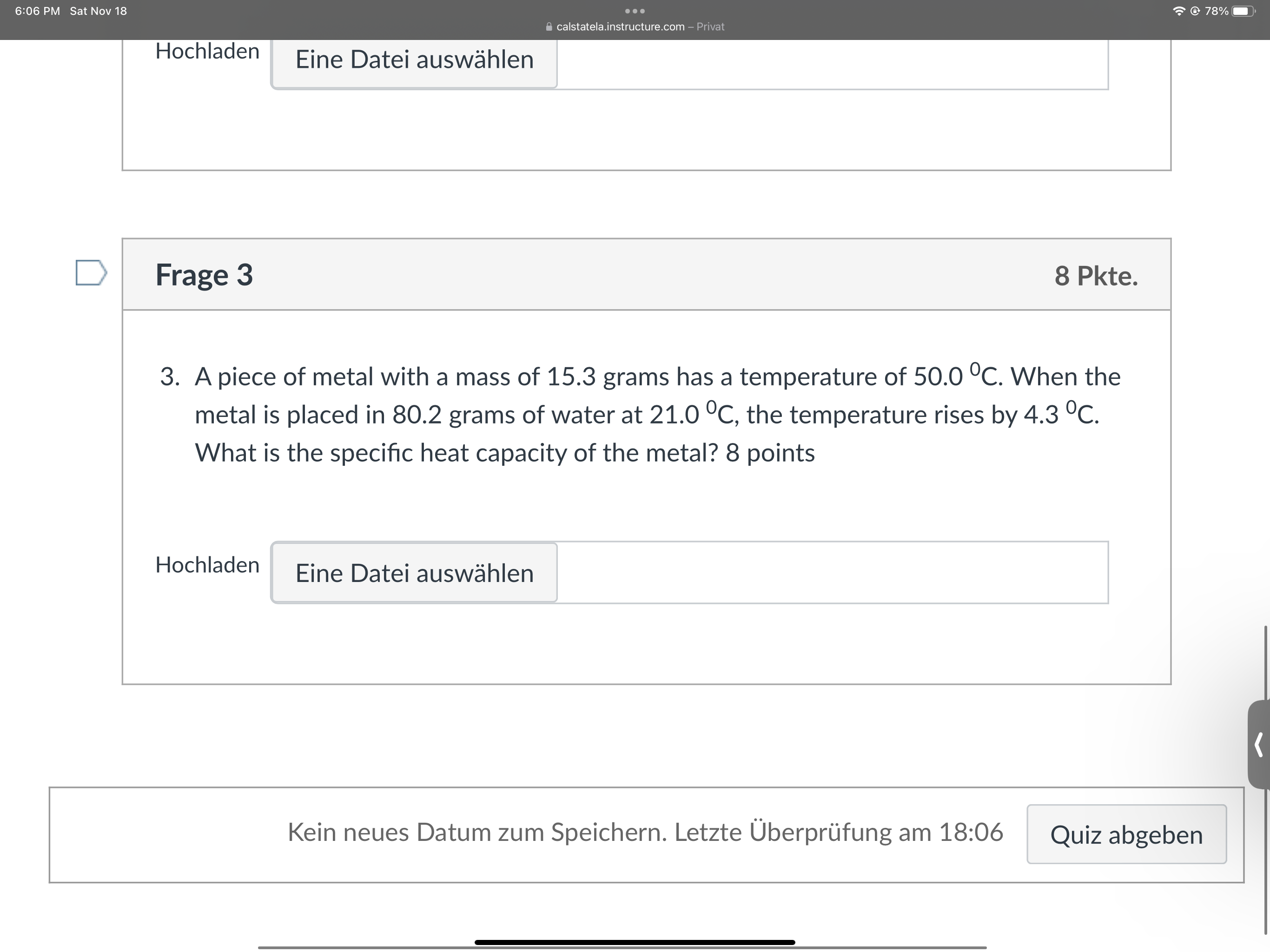
6:06 PM Sat Nov 18 . . . @ 78% calstatela.instructure.com - Privat Lic DateT adowater D Frage 2 5 Pkte. 2. How many grams of water would require 92.048 kJ of heat to raise its temperature from 34.0 C to 100.0 C? 5 points Hochladen Eine Datei auswahlen D Frage 3 8 Pkte. 3. A piece of metal with a mass of 15.3 grams has a temperature of 50.0 C. When the metal is placed in 80.2 grams of water at 21.0 C, the temperature rises by 4.3 C.6:06 PM Sat Nov 18 . . . @ 78% calstatela.instructure.com - Privat Hochladen Eine Datei auswahlen D Frage 3 8 Pkte. 3. A piece of metal with a mass of 15.3 grams has a temperature of 50.0 C. When the metal is placed in 80.2 grams of water at 21.0 C, the temperature rises by 4.3 C. What is the specific heat capacity of the metal? 8 points Hochladen Eine Datei auswahlen Kein neues Datum zum Speichern. Letzte Uberprufung am 18:06 Quiz abgeben
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


