Question: 6.(1) For the binary system n-pentanol (1) n-hexane (2), determine the activity coefficients at 313K in an equimolal mixture. The Wilson parameters are given as
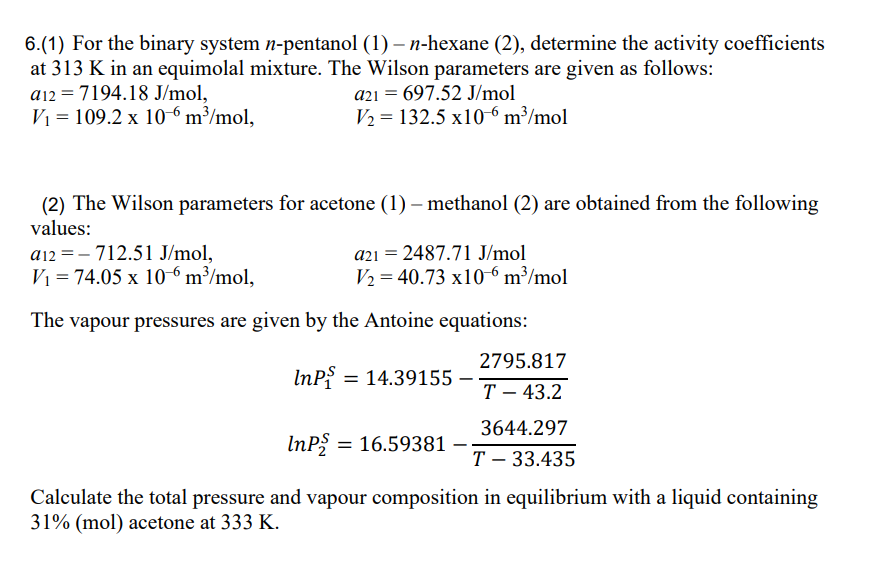
6.(1) For the binary system n-pentanol (1) n-hexane (2), determine the activity coefficients at 313K in an equimolal mixture. The Wilson parameters are given as follows: a12=7194.18J/mol,V1=109.2106m3/mol,a21=697.52J/molV2=132.5106m3/mol (2) The Wilson parameters for acetone (1)-methanol (2) are obtained from the following values: a12=712.51J/mol,V1=74.05106m3/mol,a21=2487.71J/molV2=40.73106m3/mol The vapour pressures are given by the Antoine equations: lnP1S=14.39155T43.22795.817lnP2S=16.59381T33.4353644.297 Calculate the total pressure and vapour composition in equilibrium with a liquid containing 31%(mol) acetone at 333K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


