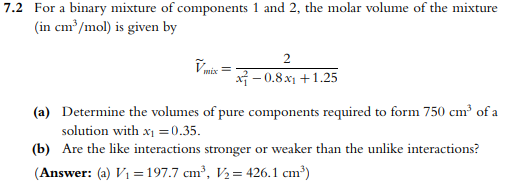
Question: 7 . 2 For a binary mixture of components 1 and 2 , the molar volume of the mixture ( in c m 3 m
For a binary mixture of components and the molar volume of the mixture
in is given by
tilde
a Determine the volumes of pure components required to form of a
solution with
b Are the like interactions stronger or weaker than the unlike interactions?
Answer: a
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


