Question: 7 : 4 5 To Do Assignment Details CHEM 5 2 4 - Electrochemical Methods Switch To Light Mode Upload to this section your answers
:
To Do
Assignment Details
CHEM Electrochemical Methods
Switch To Light Mode
Upload to this section your answers to the two activities done in class.
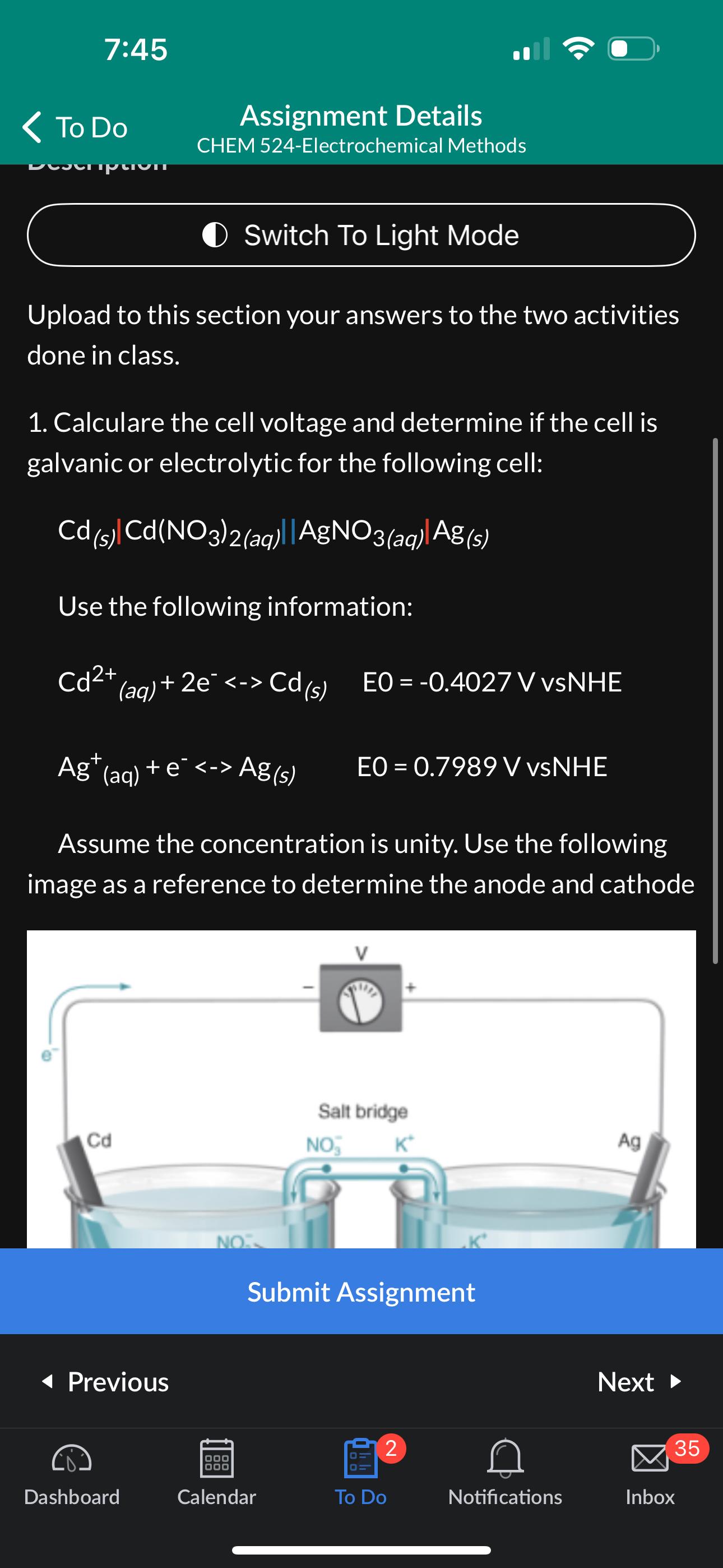
Calculare the cell voltage and determine if the cell is galvanic or electrolytic for the following cell:
Use the following information:
VvsNHE
VvNNHE
Assume the concentration is unity. Use the following image as a reference to determine the anode and cathode
Submit Assignment
Previous
Next
D
Dashboard
Calendar
Notifications
Inbox
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


