Question: 7. A fuel-oil ( 88% de C y 12% de H2-weight) is burning in a furnace producing a gas which contain CO2,O2,N2 y H2O, with
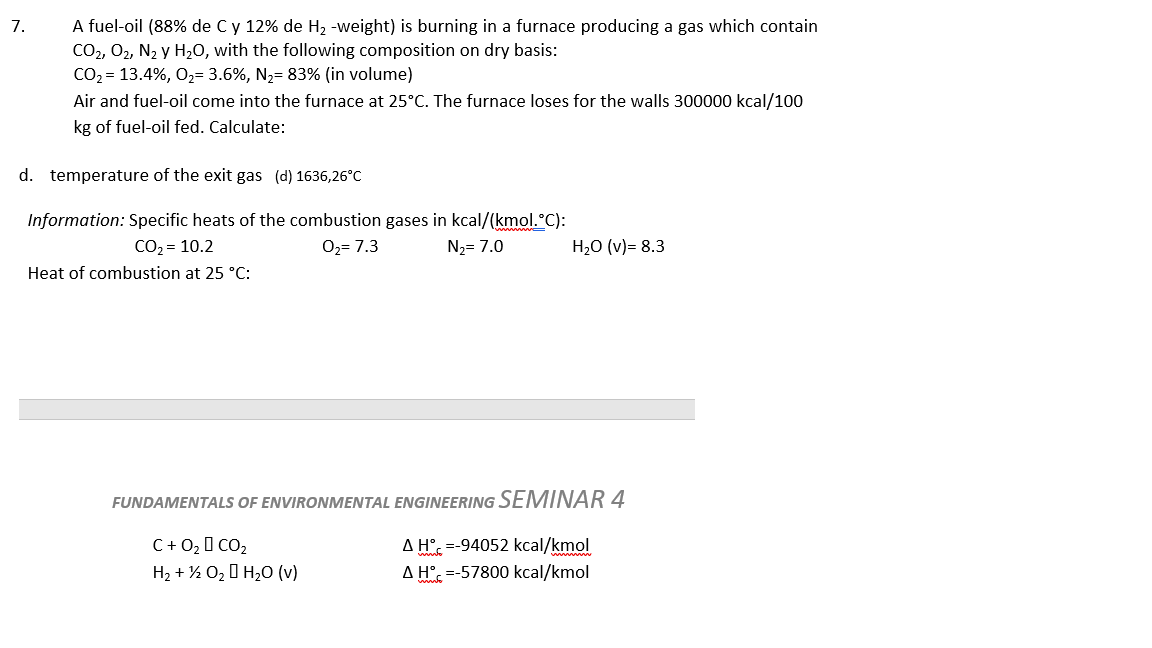
7. A fuel-oil ( 88% de C y 12% de H2-weight) is burning in a furnace producing a gas which contain CO2,O2,N2 y H2O, with the following composition on dry basis: CO2=13.4%,O2=3.6%,N2=83%(involume) Air and fuel-oil come into the furnace at 25C. The furnace loses for the walls 300000kcal/100 kg of fuel-oil fed. Calculate: d. temperature of the exit gas (d) 1636,26C Information: Specific heats of the combustion gases in kcal/(kmol..C) : CO2=10.2O2=7.3N2=7.0H2O(v)=8.3 Heat of combustion at 25C : FUNDAMENTALS OF ENVIRONMENTAL ENGINEERING SEMINAR 4 C+O2,CO2H2+1/2O2H2O(v)H=94052kcal/kmolH=57800kcal/kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


