Question: 7. Below are some standard enthalpy changes including the standard enthalpy of combustion of nitroglycerine C3H5N3O9. 76 Chapter 8: Bonding: General Concepts a) Use the
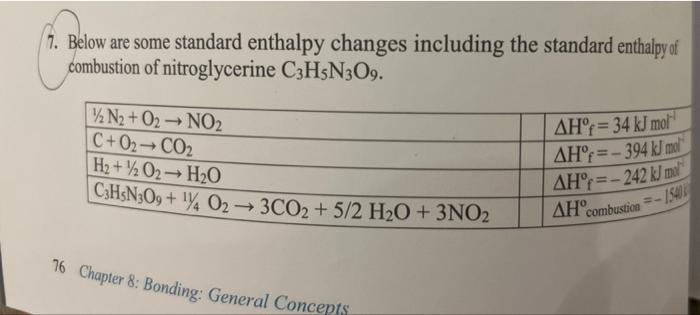
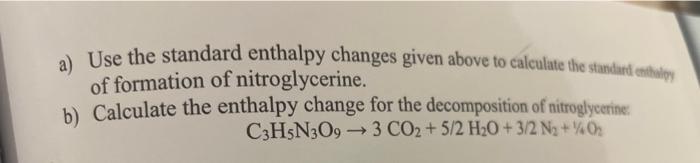
7. Below are some standard enthalpy changes including the standard enthalpy of combustion of nitroglycerine C3H5N3O9. 76 Chapter 8: Bonding: General Concepts a) Use the standard enthalpy changes given above to calculate the standart eitulipy of formation of nitroglycerine. b) Calculate the enthalpy change for the decomposition of nitroglycerine. C3H5N3O93CO2+5/2H2O+3/2N2+1/4O2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


