Question: 7 Consider the electrode reaction O + n e R . Under the conditions that C O * * = C R * * =
Consider the electrode reaction Under the conditions that and :
a Calculate the exchange current density, in
b Draw a current densityoverpotential curve for this reaction for current densities up to anodic and cathodic. Neglect masstransfer effects.
c Draw vs curves Tafel plots for the current ranges in b
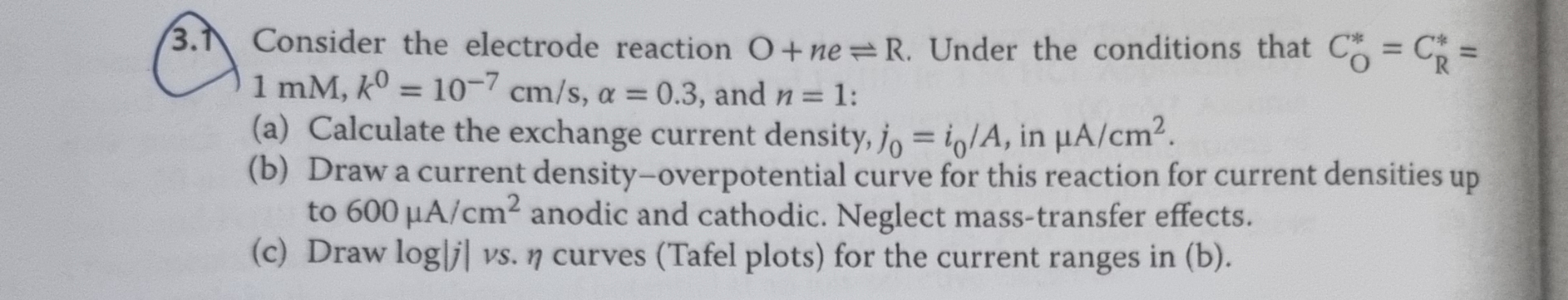
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


