Question: 7. Set up balanced reduction and oxidation reactions for the following reactions: a) Chlorine, Cl2, disproportionates to chloride, CI , and chlorate, C103 , in
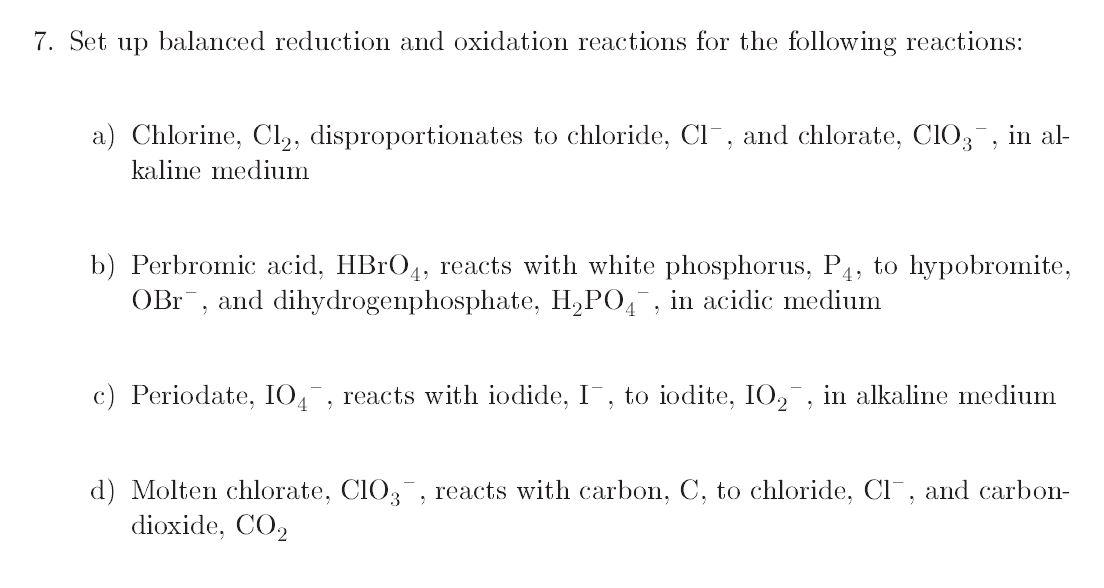
7. Set up balanced reduction and oxidation reactions for the following reactions: a) Chlorine, Cl2, disproportionates to chloride, CI , and chlorate, C103 , in al- kaline medium b) Perbromic acid, HBrO4, reacts with white phosphorus, P4, to hypobromite, OBr , and dihydrogenphosphate, H,P04, in acidic medium c) Periodate, 104 , reacts with iodide, I, to iodite, 102 , in alkaline medium d) Molten chlorate, C103 , reacts with carbon, C, to chloride, CI , and carbon- dioxide, CO2 7. Set up balanced reduction and oxidation reactions for the following reactions: a) Chlorine, Cl2, disproportionates to chloride, CI , and chlorate, C103 , in al- kaline medium b) Perbromic acid, HBrO4, reacts with white phosphorus, P4, to hypobromite, OBr , and dihydrogenphosphate, H,P04, in acidic medium c) Periodate, 104 , reacts with iodide, I, to iodite, 102 , in alkaline medium d) Molten chlorate, C103 , reacts with carbon, C, to chloride, CI , and carbon- dioxide, CO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


