Question: 7. We all know that water does not freeze at 5C and ambient pressure. Let us prove it from thermodynamic principles. Consider a heat bath
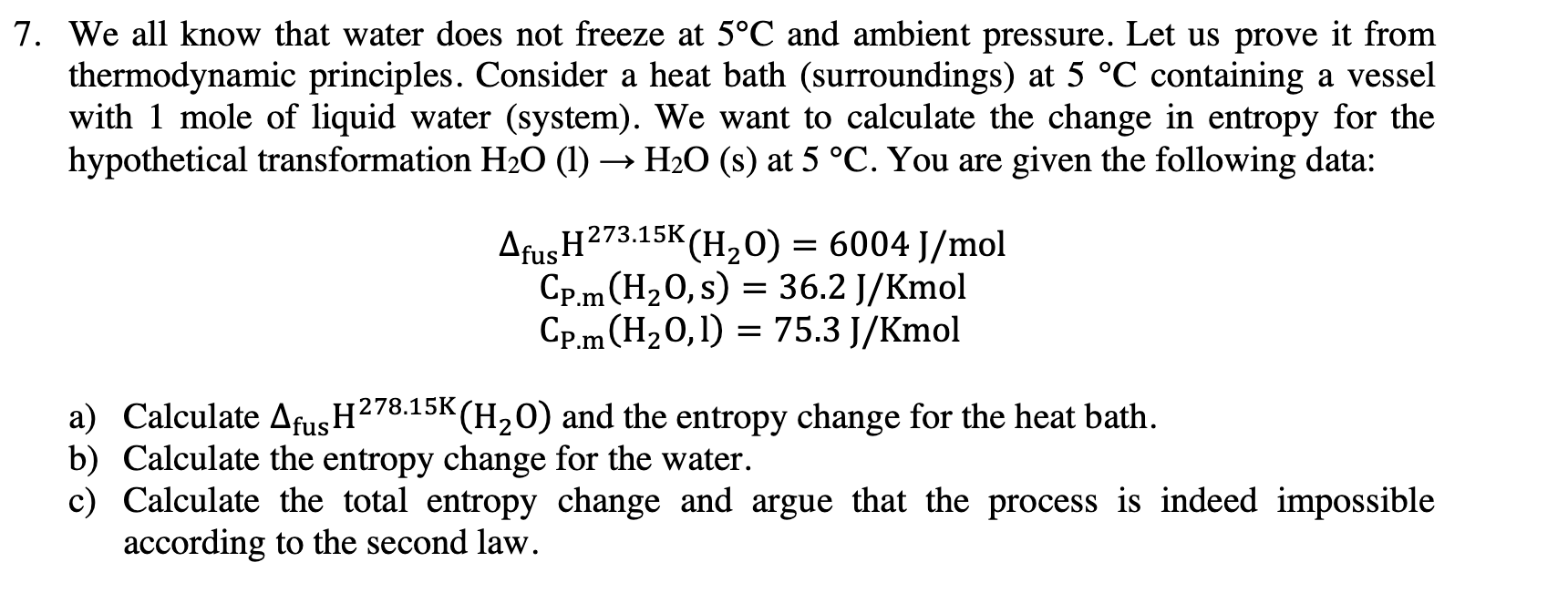
7. We all know that water does not freeze at 5C and ambient pressure. Let us prove it from thermodynamic principles. Consider a heat bath (surroundings) at 5 C containing a vessel with 1 mole of liquid water (system). We want to calculate the change in entropy for the hypothetical transformation H20 (1) > H20 (8) at 5 C. You are given the following data: Afu5H273'15K(H20) = 6004I/m01 cplmcHzo, s) = 36.21/Kmol cpm(H20, 1) = 75.31/Kmol a) Calculate AfusH278'15K(H2 O) and the entropy change for the heat bath. b) Calculate the entropy change for the water. c) Calculate the total entropy change and argue that the process is indeed impossible according to the second law
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


