Question: 7. What will be the pH of the solution when 100mL of 0.05MH3PO4 is titrated with 10mL of 0.1MNaOH solution? H3PO4Ka1=7.5103Ka2=6.2108Ka3=1.01012 a) 2.33 b) 4.21
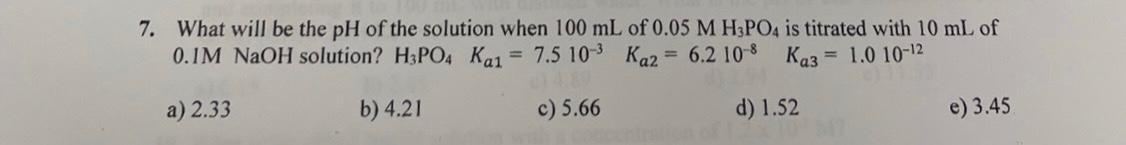
7. What will be the pH of the solution when 100mL of 0.05MH3PO4 is titrated with 10mL of 0.1MNaOH solution? H3PO4Ka1=7.5103Ka2=6.2108Ka3=1.01012 a) 2.33 b) 4.21 c) 5.66 d) 1.52 e) 3.45
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


