Question: 76 Q. Generate a Cooling curve by plotting temperature vs time data on a single graph. Plot on graphing paper or use a computer program

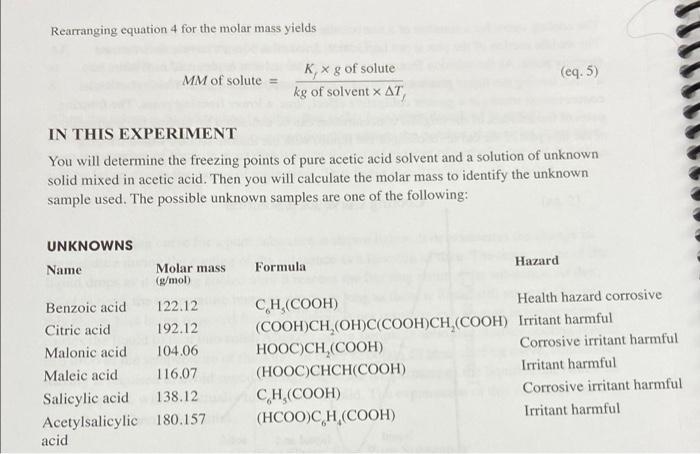
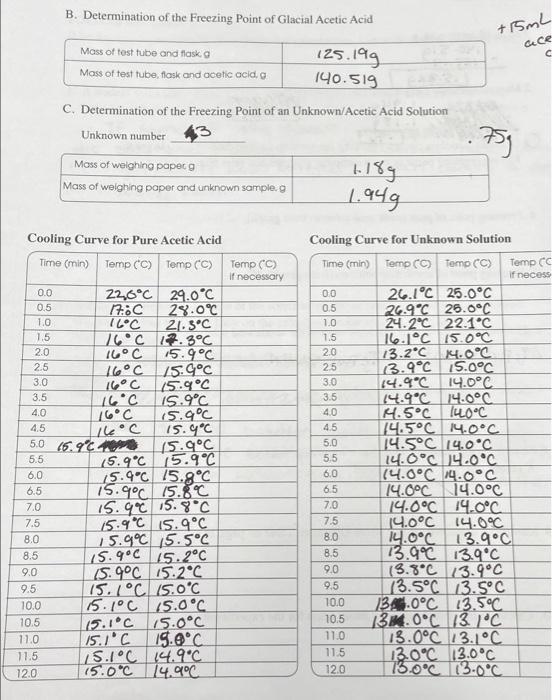
76 Q. Generate a Cooling curve by plotting temperature vs time data on a single graph. Plot on graphing paper or use a computer program and print plot. 4. Freezing point of pure acetic acid solvent (from graph), C 5. Freezing point of acetic acid solution (from graph), C 6. Determine the freezing point depression of the acetic acid solution, C ATs paresowent - ATf Pure solution 7. Determine the mass of acetic acid, kg 8. Determine the molar mass of the unknown, g/mol K (acetic acid) = 3.90 C.kg/mol 9. Identity of unknown sample (from Unknown Table on page 18) 10. Calculate the percent error in molar mass for the experiment. Rearranging equation 4 for the molar mass yields MM of solute (eq. 5) K,* g of solute kg of solvent X AT IN THIS EXPERIMENT You will determine the freezing points of pure acetic acid solvent and a solution of unknown solid mixed in acetic acid. Then you will calculate the molar mass to identify the unknown sample used. The possible unknown samples are one of the following: UNKNOWNS Name Formula Hazard Molar mass (g/mol) Benzoic acid 122.12 Citric acid 192.12 Malonic acid 104.06 Maleic acid 116.07 Salicylic acid 138.12 Acetylsalicylic 180.157 acid CH(COOH) Health hazard corrosive (COOH)CH (OH)C(COOH)CH (COOH) Irritant harmful HO0CNCH,(COOH) Corrosive irritant harmful (HOOCCHCH(COOH) Irritant harmful CH(COOH) Corrosive irritant harmful (HCOOCH(COOH) Irritant harmful B. Determination of the Freezing Point of Glacial Acetic Acid +15mL Mass of test tube and flaska Gece c Mass of test tube flask and acetic acid 125.199 190.519 C. Determination of the Freezing Point of an unknown/Acetic Acid Solution Unknown number . 753 Mass of weighing paper 9 Mass of weighing paper and unknown sample, 9 1.189 1.949 Cooling Curve for Pure Acetic Acid Time (min) Temp CC) Temp (C) Temp (C) if necessary -O Sol Cooling Curve for Unknown Solution Time (min) Temp (C) Temp (C) Temp CC if necess 00 26.1C 25.0C 0.5 26.9C 26.0C 1.0 24.2C 22.1C 1.5 16.1C 15.0C 2.0 13.2C 14.0C 25 3.9C 15.0C 3.0 14.9C 14.0C 3.5 14.9C 14.0C 40 4.5C 14C 4.5 14.5C 14.0C 5.0 14.5C 14.0C 5.5 14.0C 4.0C 6.0 (4.0C 14.0C 6.5 14.0C 14.0C 7.0 14.0C 14.0C 14.0C 14.0C 14.0C 13.9C 8.5 13.9C 13.9C 9.0 (8.8C 13.9C 9.5 (3.5C 13.5C 10.0 134.0C 13.5C 10.5 136.0C 13.1C 11.0 13.0C 13.1C 11.5 13.0C 13.0C 12.0 73.0C 13.0C 0.0 22,6C 29.0C 0.5 17.30 28.0C 1.0 16C 21.3C 1.5 16C 17.3C 2.0 16C 15.9C 2.5 16C 15.9C 3.0 16C 15.9C 3.5 16C 15.9C 4.0 16C 15.9C 4.5 L16C 15.9C 5.0 16.900 (5.9C 5.5 (5.9C 15.9C 6.0 15.9C 75.9C 6.5 75.9C 15.80 7.0 15.90 15.8C 7.5 15.9C 15.9C 8.0 15.9C 15.5C 8.5 15.9C 15.2C 9.0 15.4C 15.2C 9.5 15.7C 15.0C 10.0 15.1C 15.0C 10.5 15.1C 15.0C 11.0 15.1C 19.9C 11.5 S.1C 14.9C 12.0 15.0C 14.9C ololololololo 7.5 8.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


