Question: 8. ( 15 points) Nitrogen monoxide can be removed from gas-fired power plant emissions by reaction with methane as follows: CH4(g)+4NO(g)2N2(g)+CO2(g)+2H2O(g) Nitrogen monoxide concentration was
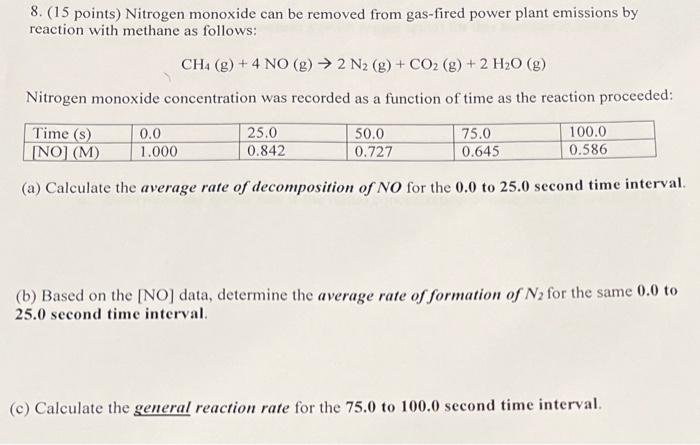
8. ( 15 points) Nitrogen monoxide can be removed from gas-fired power plant emissions by reaction with methane as follows: CH4(g)+4NO(g)2N2(g)+CO2(g)+2H2O(g) Nitrogen monoxide concentration was recorded as a function of time as the reaction proceeded: (a) Calculate the average rate of decomposition of NO for the 0.0 to 25.0 second time interval. (b) Based on the [NO] data, determine the average rate of formation of N2 for the same 0.0 to 25.0 second time interval. (c) Calculate the general reaction rate for the 75.0 to 100.0 second time interval
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


