Question: 8. 3+ Consider the following net ionic equation for a redox reaction: 16 H+ + 2 Cr0+ CH5OH 2 CO +4 Cr+ + 11 HO
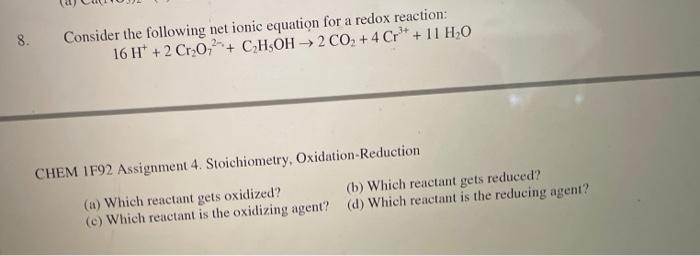
16H++2Cr2O72+C2H5OH2CO2+4Cr3+11H2O CHEM IF92 Assignment 4. Stoichiometry, Oxidation-Reduction (a) Which reactant gets oxidized? (b) Which reactant gets reduced? (c) Which reactant is the oxidizing agent? (d) Which reactant is the reducing agent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


