Question: #8) I believe its using the equation N = No*e(decay constant)t. (decay constant = the upside down y, just cant type it). if you dont
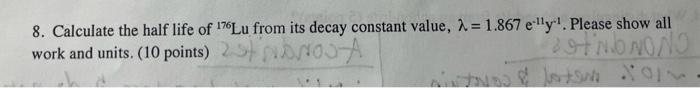
8. Calculate the half life of 176Lu from its decay constant value, =1.867e11y1. Please show all work and units. (10 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


