Question: (8%) Problem 3: Assume a certain five cent coin 1s made of pure nickel and has a mass of 4.8 grams. The atomic mass of
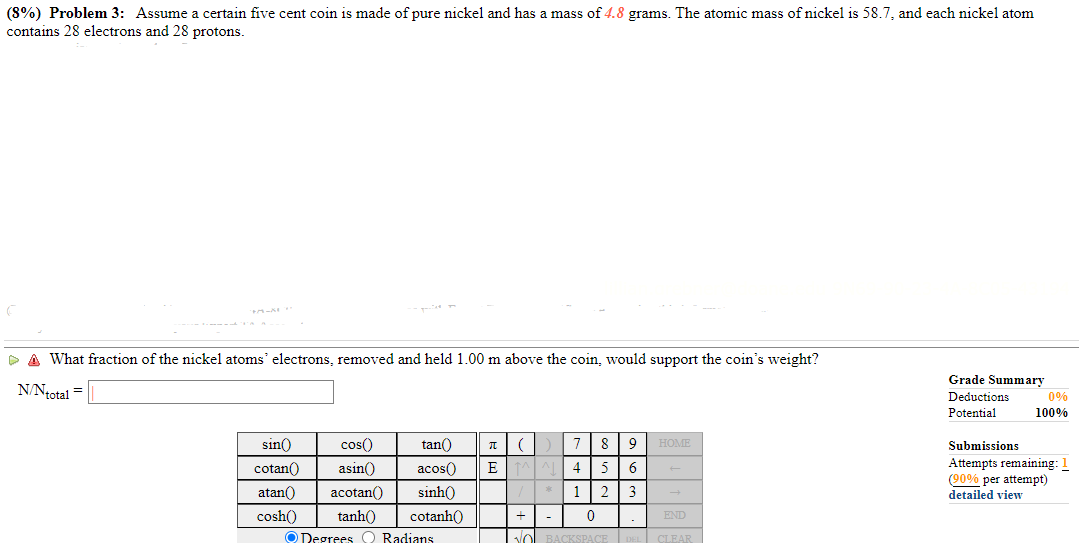
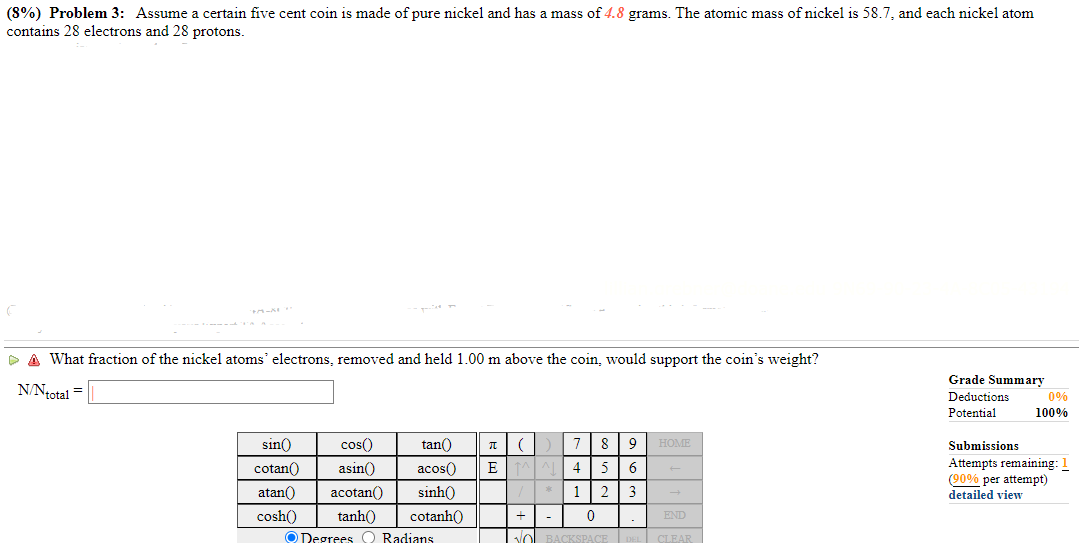
(8%) Problem 3: Assume a certain five cent coin 1s made of pure nickel and has a mass of 4.8 grams. The atomic mass of nickel 1s 58.7, and each nickel atom contains 28 electrons and 28 protons. & What fraction of the nickel atoms' electrons, removed and held 1.00 m above the coin, would support the coin's weight? N'-Ntotal = sin{) cosh() @ Neorees () Radians Grade Summary Deductions Potential 100% Submissions Attempts remaining: ( per attempt) - detailed view
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


