Question: 8 Question 8 (10 points) A compound has the empirical formula CF and has a molecular weight of 124 g/mol The true formula is: CzF
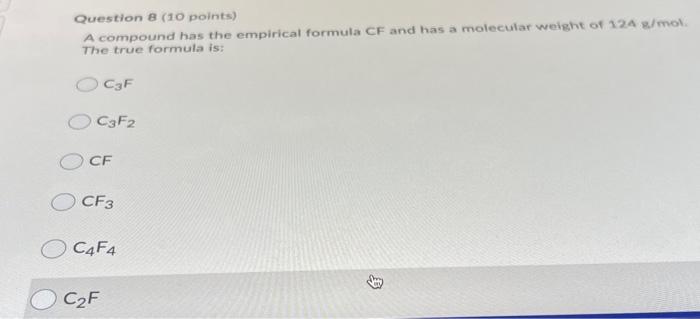
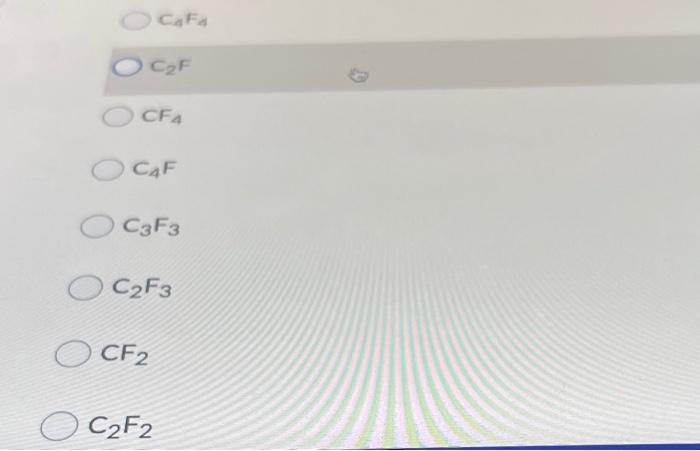
Question 8 (10 points) A compound has the empirical formula CF and has a molecular weight of 124 g/mol The true formula is: CzF CzF2 CE OCF3 C4F4 CF O CAFA OCF CFA O CAF O CF3 O CF3 CF2 C2F2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


