Question: 8) When a 0.452g sample of a compound containing only carbon, hydrogen, and nitrogen was allowed to burn in excess oxygen, it yields 0.662gCO2 and
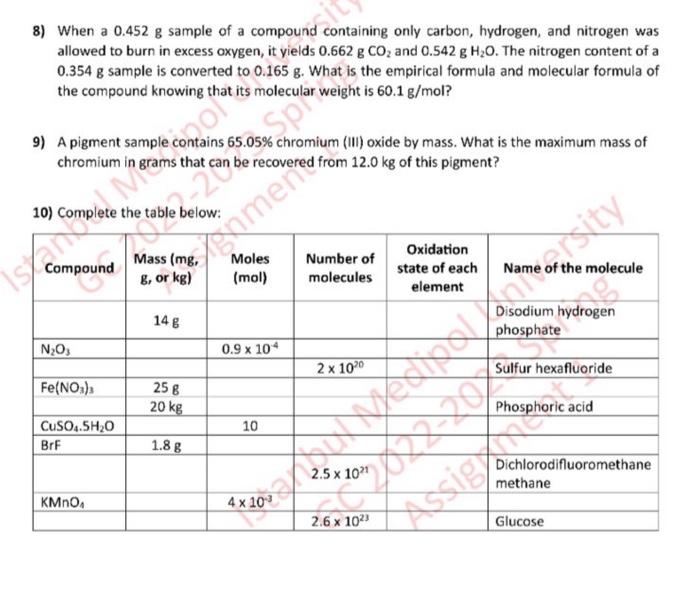
8) When a 0.452g sample of a compound containing only carbon, hydrogen, and nitrogen was allowed to burn in excess oxygen, it yields 0.662gCO2 and 0.542gH2O. The nitrogen content of a 0.354g sample is converted to 0.165g. What is the empirical formula and molecular formula of the compound knowing that its molecular weight is 60.1g/mol ? 9) A pigment sample contains 65.05% chromium (III) oxide by mass. What is the maximum mass of chromium in grams that can be recovered from 12.0kg of this pigment? 10) Complete the table below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


