Question: 8.36 Using the data below, calculate the partial molar enthalpies of 1-propanol and water as a function of composition at both 25C and 50C. 25C
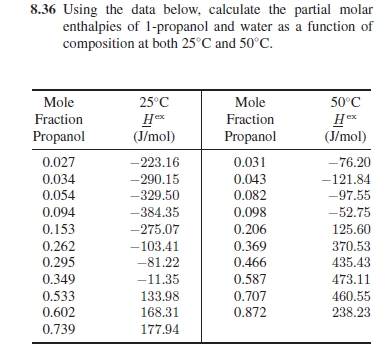
8.36 Using the data below, calculate the partial molar enthalpies of 1-propanol and water as a function of composition at both 25C and 50C. 25C 50C Hox Hex (J/mol) Mole Fraction Propanol 0.027 0.034 0.054 0.094 0.153 0.262 0.295 0.349 0.533 0.602 0.739 (J/mol) -223.16 - 290.15 -329.50 -384.35 -275.07 -103.41 -81.22 -11.35 133.98 168.31 177.94 Mole Fraction Propanol 0.031 0.043 0.082 0.098 0.206 0.369 0.466 0.587 0.707 0.872 -76.20 -121.84 -97.55 -52.75 125.60 370.53 435.43 473.11 460.55 238.23
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


