Question: 9 2 CO) + O2(e) ----> 2 CO2) (OR 2 CO(S) + O2(g) right arrow 2 CO2(8) Use the following conversions: 1 mol - MW
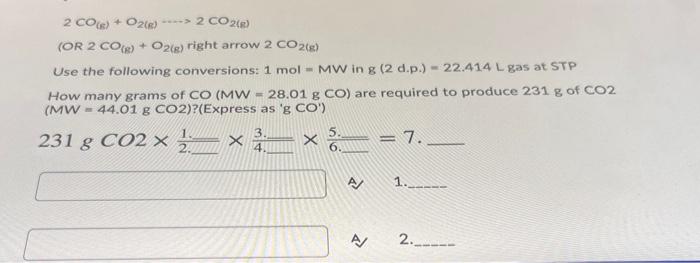
2 CO) + O2(e) ----> 2 CO2) (OR 2 CO(S) + O2(g) right arrow 2 CO2(8) Use the following conversions: 1 mol - MW in g (2 d.d.) - 22.414 L gas at STP How many grams of CO (MW-28.01 g CO) are required to produce 231 g of CO2 (MW - 44.01 g CO2)?(Express as 'g CO") - 8 231 g CO2 x 2x 7. 4 1 A 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


