Question: 9. (20 pts) The net potential energy between two adjacent ions, Ex. may be represented by the equation shown below, where A,B and n are
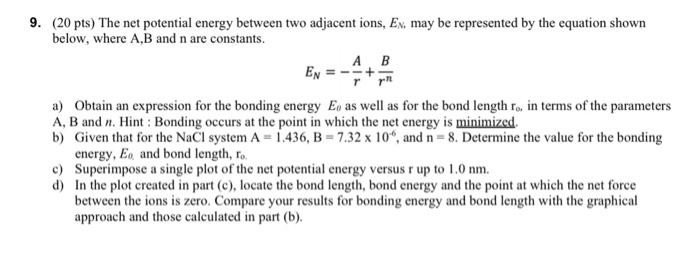
9. (20 pts) The net potential energy between two adjacent ions, Ex. may be represented by the equation shown below, where A,B and n are constants. EN a) Obtain an expression for the bonding energy E, as well as for the bond length ro, in terms of the parameters A, B and n. Hint: Bonding occurs at the point in which the net energy is minimized b) Given that for the NaCl system A = 1.436, B = 7.32 x 10", and n = 8. Determine the value for the bonding energy, Eo and bond length, Co. c) Superimpose a single plot of the net potential energy versus r up to 1.0 nm. d) In the plot created in part (c), locate the bond length, bond energy and the point at which the net force between the ions is zero. Compare your results for bonding energy and bond length with the graphical approach and those calculated in part (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


