Question: 9) How do you expect the cysteine thiol will be affected by hydrogen bonding to the side chain of a glutamic acid in the
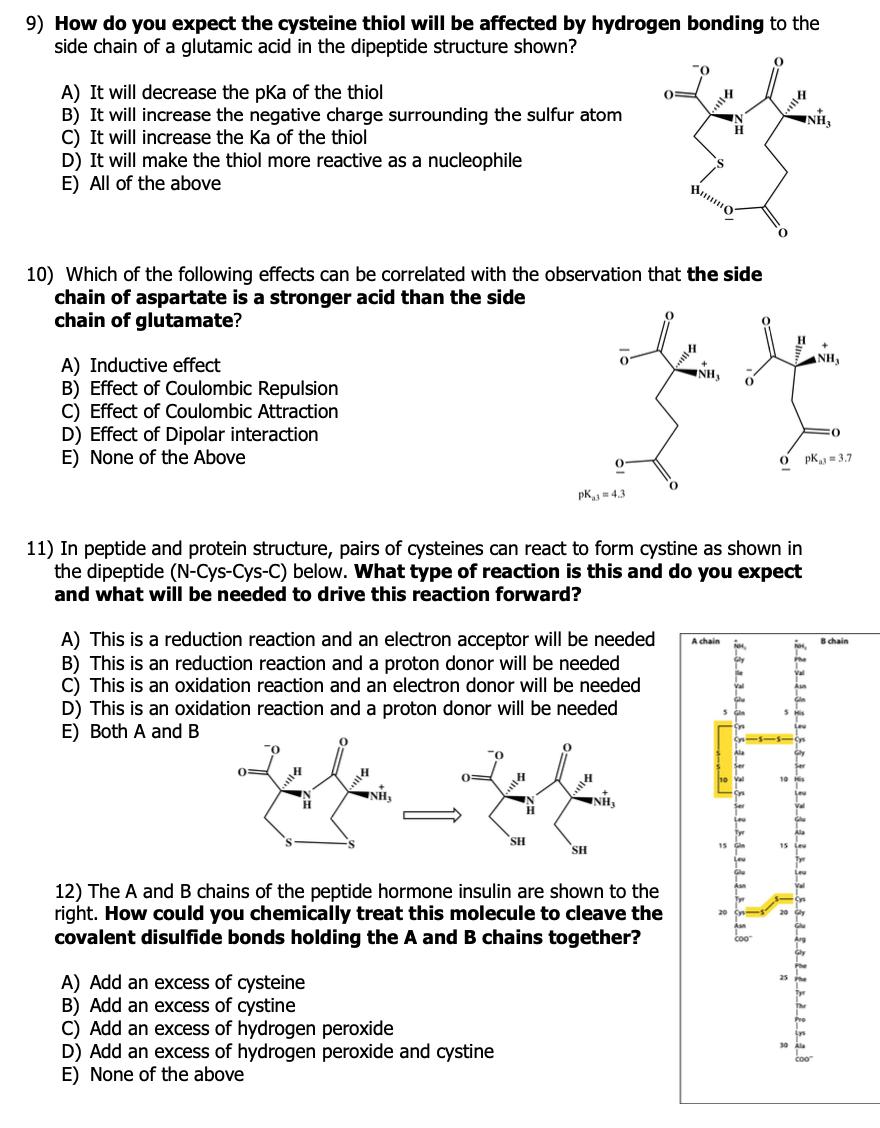
9) How do you expect the cysteine thiol will be affected by hydrogen bonding to the side chain of a glutamic acid in the dipeptide structure shown? A) It will decrease the pka of the thiol B) It will increase the negative charge surrounding the sulfur atom C) It will increase the Ka of the thiol D) It will make the thiol more reactive as a nucleophile E) All of the above 10) Which of the following effects can be correlated with the observation that the side chain of aspartate is a stronger acid than the side chain of glutamate? NH, A) Inductive effect B) Effect of Coulombic Repulsion C) Effect of Coulombic Attraction D) Effect of Dipolar interaction E) None of the Above NH, pk = 3.7 O. pK = 4.3 11) In peptide and protein structure, pairs of cysteines can react to form cystine as shown in the dipeptide (N-Cys-Cys-C) below. What type of reaction is this and do you expect and what will be needed to drive this reaction forward? A) This is a reduction reaction and an electron acceptor will be needed B) This is an reduction reaction and a proton donor will be needed C) This is an oxidation reaction and an electron donor will be needed D) This is an oxidation reaction and a proton donor will be needed E) Both A and B A chain Bchain 0= SH SH 12) The A and B chains of the peptide hormone insulin are shown to the right. How could you chemically treat this molecule to cleave the covalent disulfide bonds holding the A and B chains together? A) Add an excess of cysteine B) Add an excess of cystine C) Add an excess of hydrogen peroxide D) Add an excess of hydrogen peroxide and cystine E) None of the above
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


