Question: 9. please help will rate you! Consider the reaction described by the equation C2H4Br2(aq)+3I(aq)C2H4(g)+2Br(aq)+I3(aq) The rate law is rate=k[C2H4Br2][I] where k=0.00417M1+s1, What are the missing
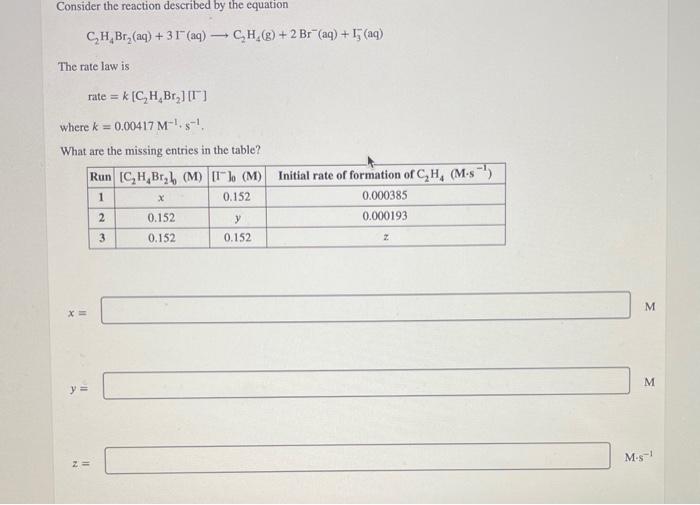
Consider the reaction described by the equation C2H4Br2(aq)+3I(aq)C2H4(g)+2Br(aq)+I3(aq) The rate law is rate=k[C2H4Br2][I] where k=0.00417M1+s1, What are the missing entries in the table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


