Question: 9. Which statement below regarding rate laws and rate constants is true? A. The rate constant is independent of temperature. B. The units of the
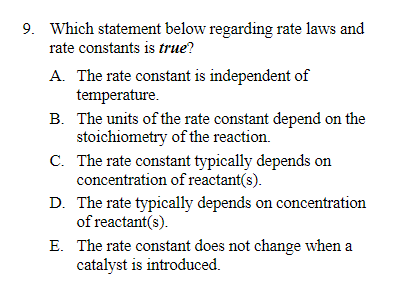
9. Which statement below regarding rate laws and rate constants is true? A. The rate constant is independent of temperature. B. The units of the rate constant depend on the stoichiometry of the reaction. C. The rate constant typically depends on concentration of reactant(s). D. The rate typically depends on concentration of reactant(s). E. The rate constant does not change when a catalyst is introduced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


