Question: 9.2 Orbitals which result from solving the Schrdinger Wave Equation can be represented by electron cloud pictures. The dots in these pictures represent the probability
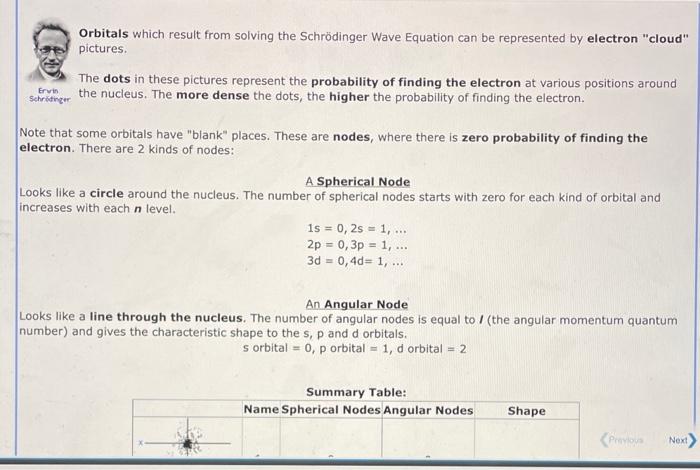
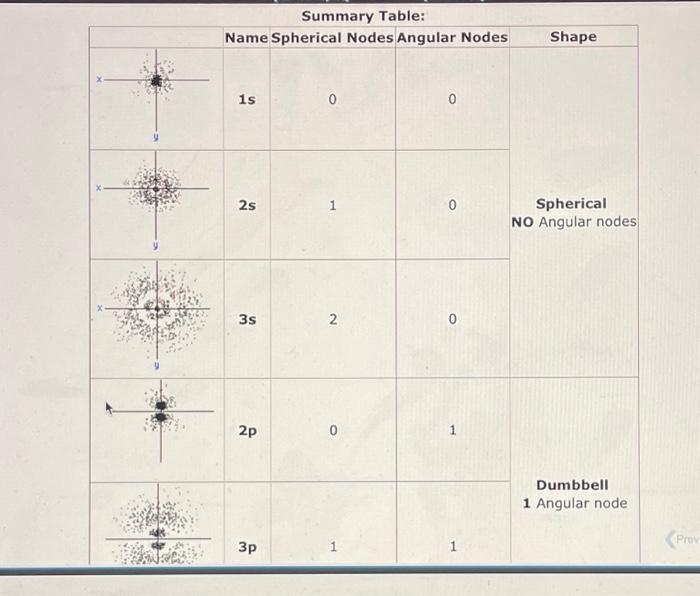
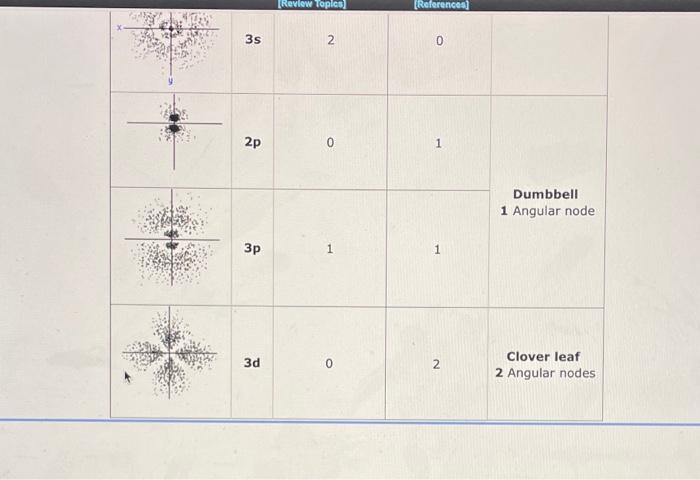
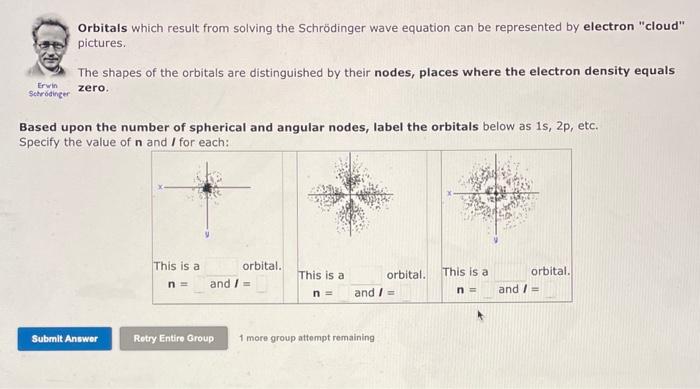
Orbitals which result from solving the Schrdinger Wave Equation can be represented by electron "cloud" pictures. The dots in these pictures represent the probability of finding the electron at various positions around secruitinger the nucleus. The more dense the dots, the higher the probability of finding the electron. Note that some orbitals have "blank" places. These are nodes, where there is zero probability of finding the electron. There are 2 kinds of nodes: A Spherical Node Looks like a circle around the nucleus. The number of spherical nodes starts with zero for each kind of orbital and increases with each n level. 1s=0,2s=1,2p=0,3p=1,3d=0,4d=1, An Angular Node Looks like a line through the nucleus. The number of angular nodes is equal to I (the angular momentum quantum number) and gives the characteristic shape to the s, p and d orbitals. sorbital=0,porbital=1,dorbital=2 Summary Table: Orbitals which result from solving the Schrdinger wave equation can be represented by electron "cloud" pictures. The shapes of the orbitals are distinguished by their nodes, places where the electron density equals setrowinger zero. Based upon the number of spherical and angular nodes, label the orbitals below as 1s, 2p, etc. Specify the value of n and I for each
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


