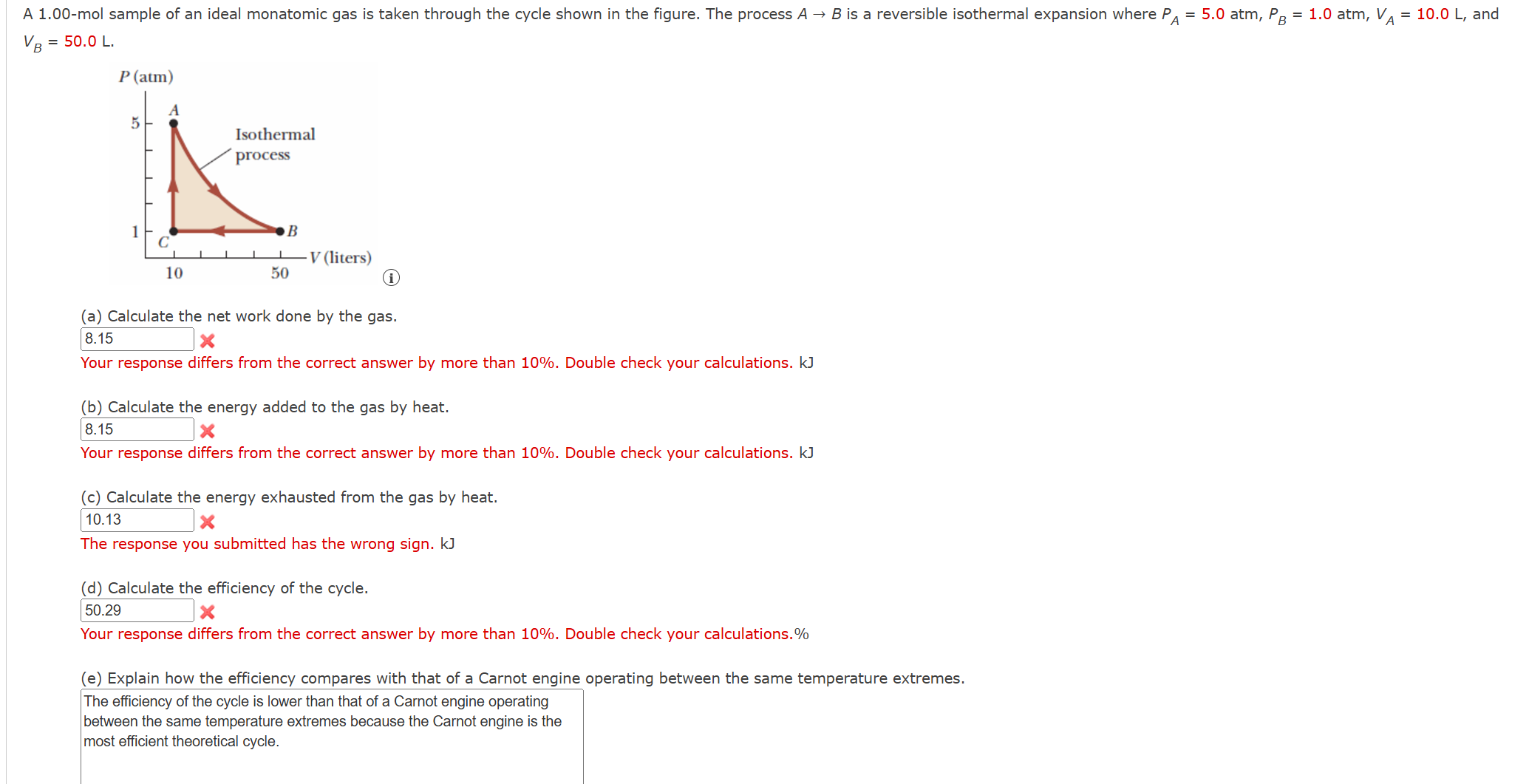
Question: A ( 1 . 0 0 - mathrm { mol } ) sample of an ideal monatomic gas is taken through the
A mathrmmol sample of an ideal monatomic gas is taken through the cycle shown in the figure. The process A rightarrow B is a reversible isothermal expansion where PA atm, PB atm, VAmathrm~L and VBmathrm~L
a Calculate the net work done by the gas.
Your response differs from the correct answer by more than Double check your calculations. kJ
b Calculate the energy added to the gas by heat.
Your response differs from the correct answer by more than Double check your calculations. kJ
c Calculate the energy exhausted from the gas by heat.
The response you submitted has the wrong sign. kJ
d Calculate the efficiency of the cycle.
Your response differs from the correct answer by more than Double check your calculations.
e Explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes.
The efficiency of the cycle is lower than that of a Carnot engine operating between the same temperature extremes because the Carnot engine is the most efficient theoretical cycle.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


