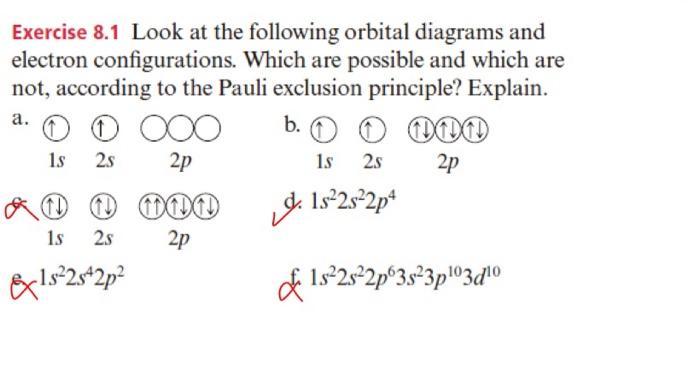
Question: r Exercise 8.1 Look at the following orbital diagrams and electron configurations. Which are possible and which are not, according to the Pauli exclusion principle?
 r
r
Exercise 8.1 Look at the following orbital diagrams and electron configurations. Which are possible and which are not, according to the Pauli exclusion principle? Explain. a. 0 000 1s 25 2p 1 1 001 2p 1s 2s &1s2s+2p b. 0 0 000 1s 2s 2p 1s2s2p4 1s 2s-2p 3s3p103d0
Step by Step Solution
There are 3 Steps involved in it
Pauli exclusion principle states that an orbital can contain maximum of two electr... View full answer

Get step-by-step solutions from verified subject matter experts


