Question: A 2.129g sample of a solid mixture containing only potassium carbonate (MM=138.2058g/mol) and potassium bicarbonate ( MM=100.1154g/mol ) is dissolved in distilled water. A volume
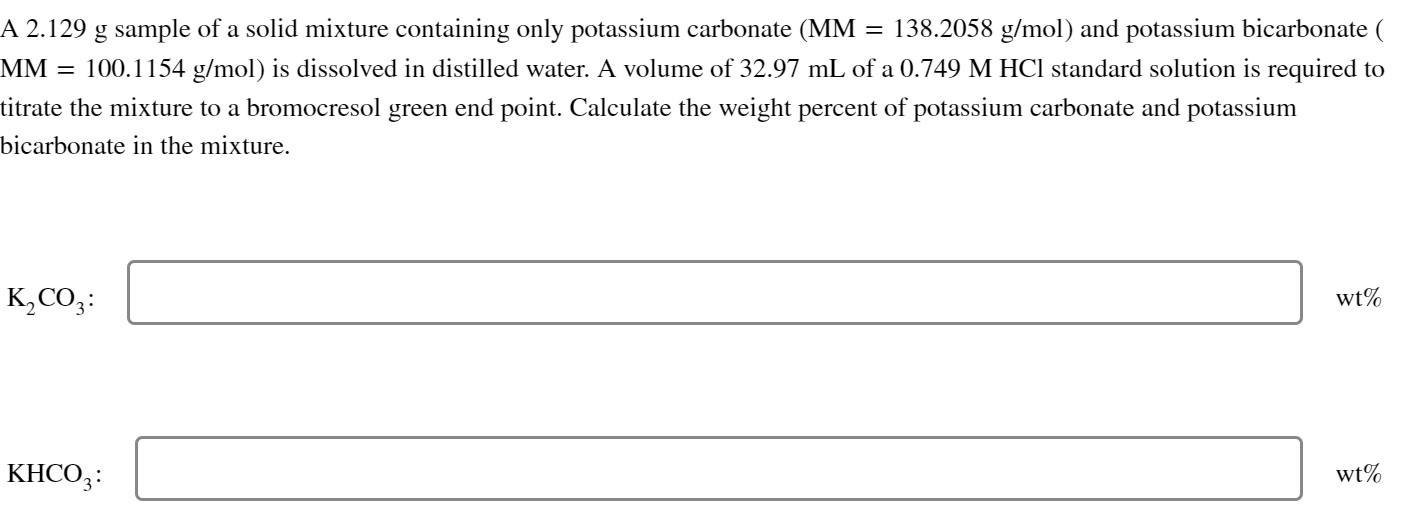
A 2.129g sample of a solid mixture containing only potassium carbonate (MM=138.2058g/mol) and potassium bicarbonate ( MM=100.1154g/mol ) is dissolved in distilled water. A volume of 32.97mL of a 0.749MHCl standard solution is required to titrate the mixture to a bromocresol green end point. Calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


