Question: A 260. mL. sample of a sugar solution containing 1.68g of the sugar has an osmotic pressure of 30.3mmHg at 34.9 o C. What is
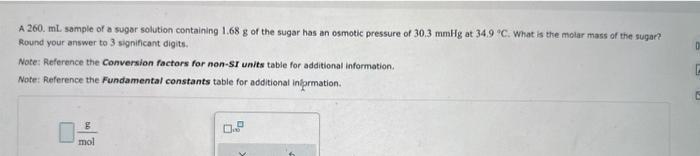
A 260. mL. sample of a sugar solution containing 1.68g of the sugar has an osmotic pressure of 30.3mmHg at 34.9 o C. What is the melar mass of the sugae? Round your answer te 3 significant digits. Note: Reference the Conversion factors for non-SI units table for additional information. Note: Reference the Fundamental constants table for additional information
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


