Question: A) 3 B) 5 C) 8 D) 9 E) 2 Q2: A sample of 5g of Ba(OH)2 is dissolved in enough water to make 1L
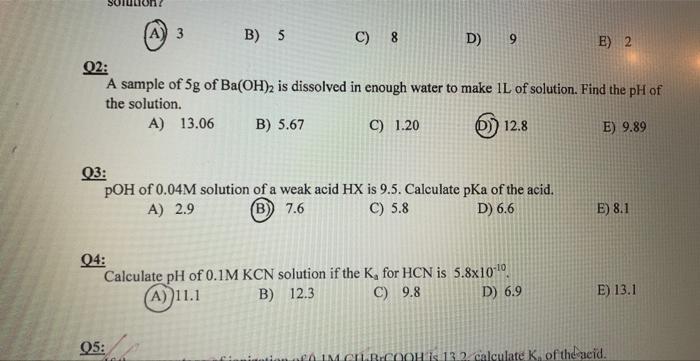
A) 3 B) 5 C) 8 D) 9 E) 2 Q2: A sample of 5g of Ba(OH)2 is dissolved in enough water to make 1L of solution. Find the pH of the solution. A) 13.06 B) 5.67 C) 1.20 (D)) 12.8 E) 9.89 Q3: pOH of 0.04M solution of a weak acid HX is 9.5. Calculate pKa of the acid. A) 2.9 (B) 7.6 C) 5.8 D) 6.6 E) 8.1 Q4: Calculate pH of 0.1MKCN solution if the Ka for HCN is 5.81010. (A) 11.1 B) 12.3 C) 9.8 D) 6.9 E) 13.1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


