Question: (a) (4 points) Calculate the binding constant for the formation of a hydrogen-bond complex between molecules 1 and 2 in chloroform at 298 K. The
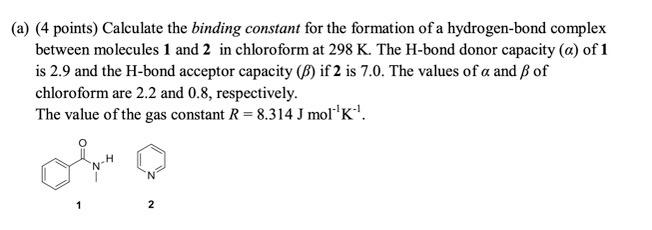
(a) (4 points) Calculate the binding constant for the formation of a hydrogen-bond complex between molecules 1 and 2 in chloroform at 298 K. The H-bond donor capacity (a) of 1 is 2.9 and the H-bond acceptor capacity (B) if 2 is 7.0. The values of a and of chloroform are 2.2 and 0.8, respectively. The value of the gas constant R= 8.314 J mol'k! 1 2 (a) (4 points) Calculate the binding constant for the formation of a hydrogen-bond complex between molecules 1 and 2 in chloroform at 298 K. The H-bond donor capacity (a) of 1 is 2.9 and the H-bond acceptor capacity (B) if 2 is 7.0. The values of a and of chloroform are 2.2 and 0.8, respectively. The value of the gas constant R= 8.314 J mol'k! 1 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


