Question: In general, it is very difficult to rotate around a double bond. The barrier of rotation for most carbon-carbon double bonds is about 66 kcal/mol.
In general, it is very difficult to rotate around a double bond. The barrier of rotation for most carbon-carbon double bonds is about 66 kcal/mol. The barrier for rotation about the central bond in molecule A, however, is only about 13 kcal/mol. Explain why the rotation about this bond is considerably easier than most other double bonds.
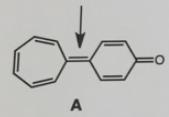
A
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
The molecule shown is a benzophenone derivative with a structure where the carboncarbon double bond ... View full answer

Get step-by-step solutions from verified subject matter experts


