Question: A 52 g ice cube at -20 C is placed into a pot on the stove. Calculate how much heat it would take to turn
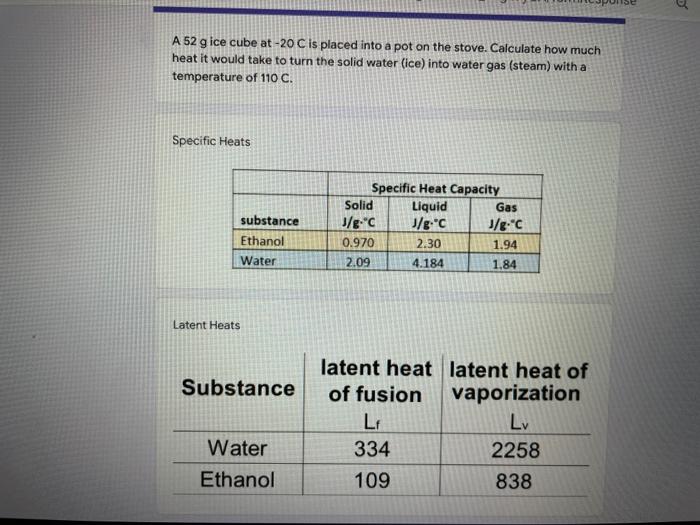
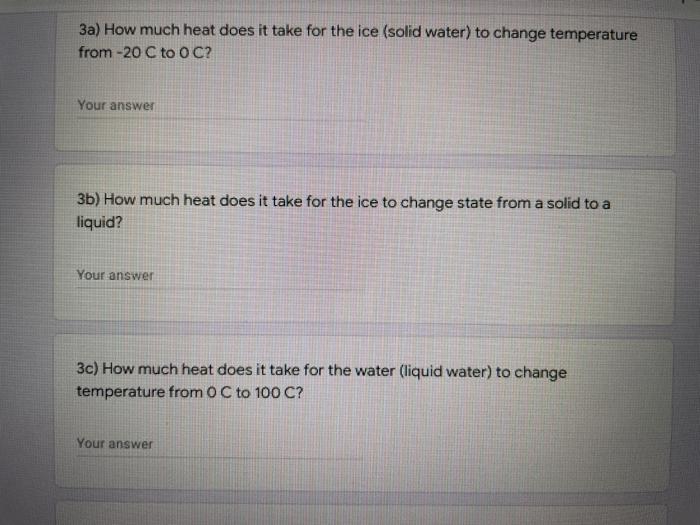
A 52 g ice cube at -20 C is placed into a pot on the stove. Calculate how much heat it would take to turn the solid water (ice) into water gas (steam) with a temperature of 110 C. Specific Heats substance Ethanol Water Specific Heat Capacity Solid Liquid Gas J/g."C J/g C J/ec 0.970 2.30 1.94 2.09 4.184 1.84 Latent Heats Substance latent heat latent heat of of fusion vaporization LF 334 2258 109 838 Water Ethanol 3a) How much heat does it take for the ice (solid water) to change temperature from -20 C to O C? Your answer 3b) How much heat does it take for the ice to change state from a solid to a liquid? Your answer 3c) How much heat does it take for the water (liquid water) to change temperature from 0 C to 100 C? Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


