Question: A 9 2 . 2 m L sample of 1 . 0 0 MNaOH is mixed with 4 6 . 1 m L of 1
A sample of MNaOH is mixed with of in a large Styrofoam coffee cup; the cup is fitted with a lid through which passes a calibrated thermometer. The temperature of each solution before mixing is After adding the NaOH solution to the coffee cup and stirring the mixed solutions with the thermometer, the maximum temperature measured is Assume that the density of the mixed solutions is that the specific heat of the mixed solutions is and that no heat is lost to the surroundings.
th attempt
Part point
See Periodic Table
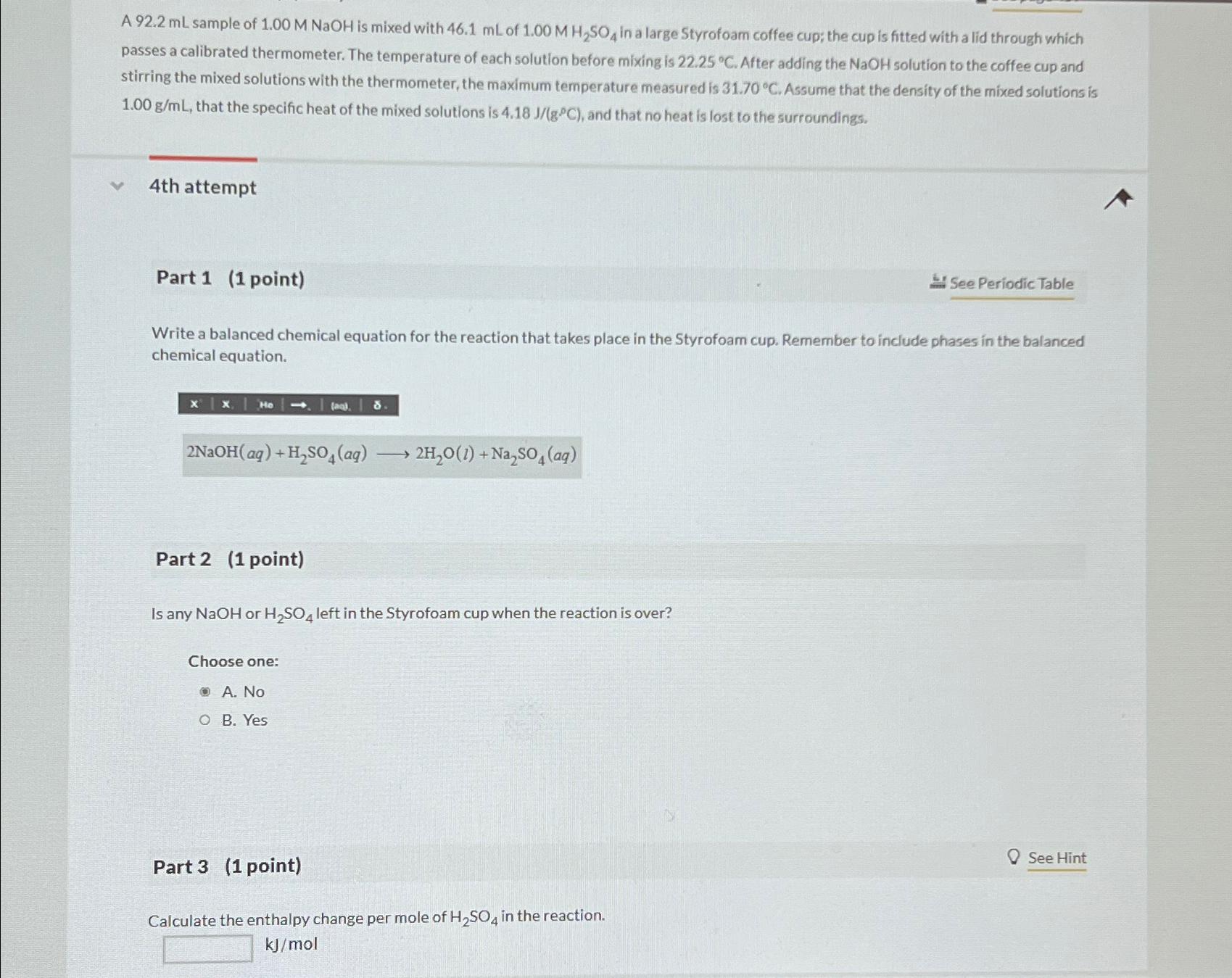
Write a balanced chemical equation for the reaction that takes place in the Styrofoam cup. Remember to include phases in the balanced chemical equation.
tableNaOHlongrightarrow
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


