Question: a ) A coal - fired power station is an application of a heat engine. It uses heat transfer from burning coal, does useful work
a A coalfired power station is an application of a heat engine. It uses heat transfer from burning coal, does useful work by turning a turbine, and exhausts heat to a cold reservoir.
Derive an expression for the thermal efficiency of the power station in terms of heat energy received from burning coal and heat transfer to the cold reservoir
Explain first and second laws of thermodynamics when you apply them in deriving the above expression. Assume a cyclical process is used in the power station.
Using your derived expressions calculate the heat transferred from coal and heat transferred to the environment in a MW coal power station operates with a thermal efficiency of
b Explain how you apply first law of thermodynamics to obtain the steady flow energy equation SFEE for an open system. Use the SFEE to produce the energy transfer equation for a boiler in terms of enthalpy changes assuming potential and kinetic energy changes are negligible.
Use the SFEE to a boiler designed to work at bars and evaporates of water. The inlet water to the boiler has a temperature of and at exit the steam is dry. The flow velocity at inlet is and at exit and the exit is m above the elevation at entrance. Determine the quantity of heat required in kW Explain the significance of changes in kinetic and potential energy on the overall result. Compare using the expression derived in Steam tables are provided with the assignment.
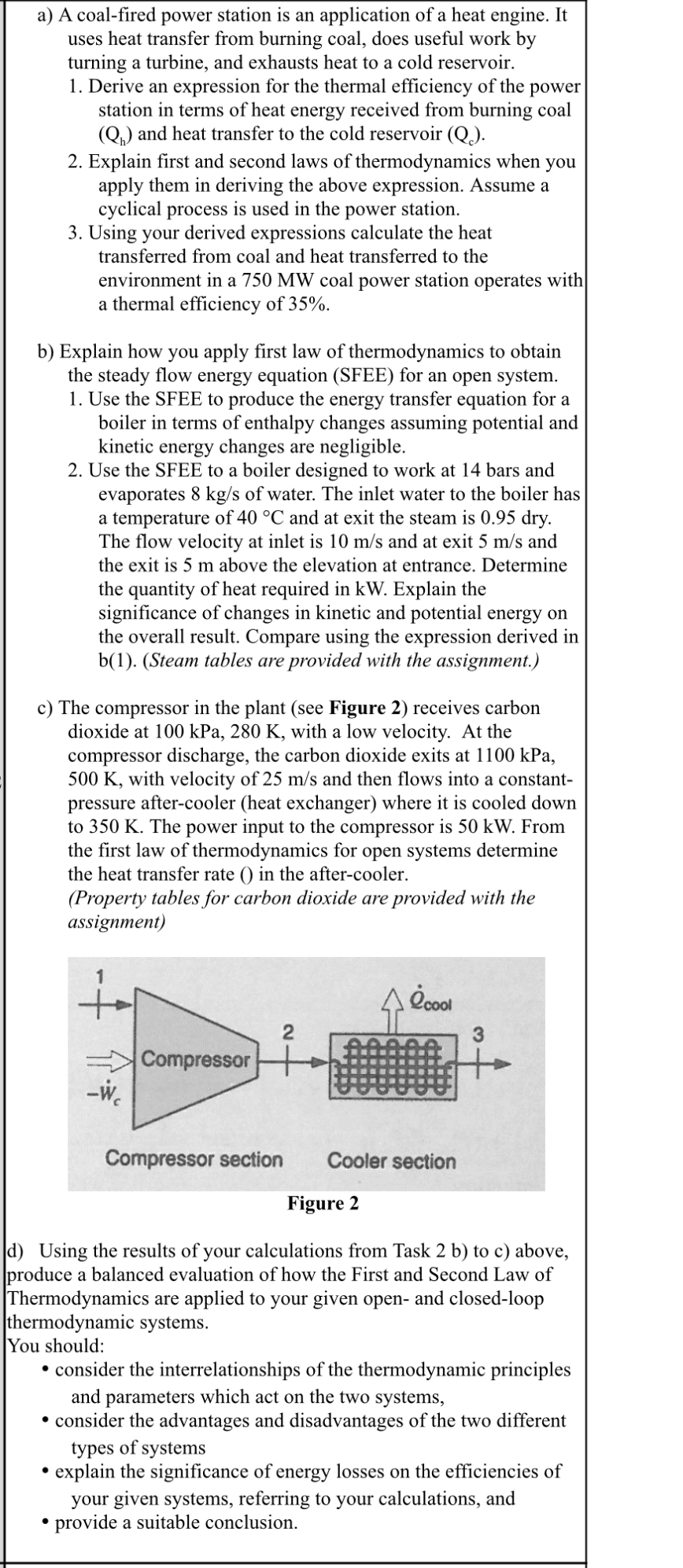
c The compressor in the plant see Figure receives carbon dioxide at kPa, with a low velocity. At the compressor discharge, the carbon dioxide exits at kPa K with velocity of and then flows into a constantpressure aftercooler heat exchanger where it is cooled down to K The power input to the compressor is kW From the first law of thermodynamics for open systems determine the heat transfer rate in the aftercooler. Property tables for carbon dioxide are provided with the assignment
d Using the results of your calculations from Task b to c above, produce a balanced evaluation of how the First and Second Law of Thermodynamics are applied to your given open and closedloop thermodynamic systems.
You should:
consider the interrelationships of the thermodynamic principles and parameters which act on the two systems,
consider the advantages and disadvantages of the two different types of systems
explain the significance of energy losses on the efficiencies of your given systems, referring to your calculations, and provide a suitable conclusion.
I need the answer for last question D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


