Question: a A sealed and initially empty container has a piston that allows for the volume to be adjustable. The volume is set to 2.50 L
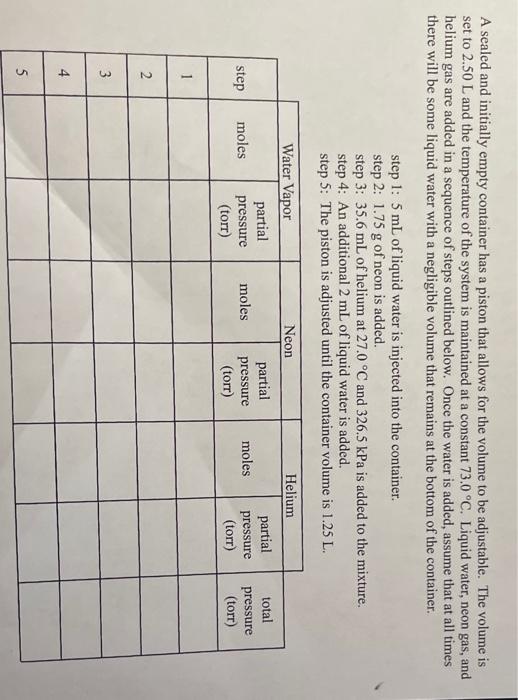
a A sealed and initially empty container has a piston that allows for the volume to be adjustable. The volume is set to 2.50 L and the temperature of the system is maintained at a constant 73.0 C. Liquid water, neon gas, and helium gas are added in a sequence of steps outlined below. Once the water is added, assume that at all times there will be some liquid water with a negligible volume that remains at the bottom of the container. step 1: 5 mL of liquid water is injected into the container step 2: 1.75 g of neon is added. step 3: 35.6 mL of helium at 27.0 C and 326.5 kPa is added to the mixture. step 4: An additional 2 mL of liquid water is added. step 5: The piston is adjusted until the container volume is 1.25 L. Helium Water Vapor partial moles pressure (torr) Neon partial moles pressure (torr) moles partial pressure (torr) total pressure (torr) step 1 N 3 4 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


