Question: a) Assisted by molecular structures, determine the valence electron (VE) for the transition metals in the following complexes using the ionic and covalent counting conventions.
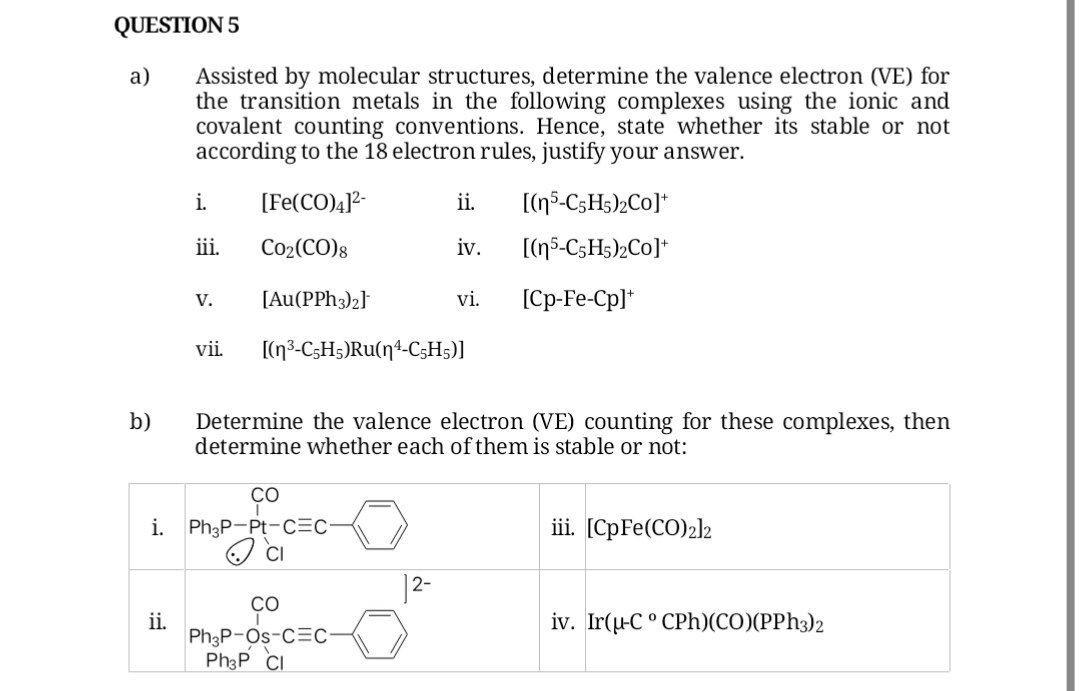
a) Assisted by molecular structures, determine the valence electron (VE) for the transition metals in the following complexes using the ionic and covalent counting conventions. Hence, state whether its stable or not according to the 18 electron rules, justify your answer. i. [Fe(CO)4]2 ii. [(5C5H5)2Co+]+ iii. Co2(CO)8 iv. [(5C5H5)2Co+ v. [Au(PPh3)2] vi. [CpFeCp]+ vii. [(3C5H5)Ru(4C5H5)] b) Determine the valence electron (VE) counting for these complexes, then determine whether each of them is stable or not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


