Question: A) At 25C temperature with Cu2+ ion catalyst (CuSO4) at concentrations of 5ppm and certain acidity concentrations, a value of 0.021 days 1 was obtained
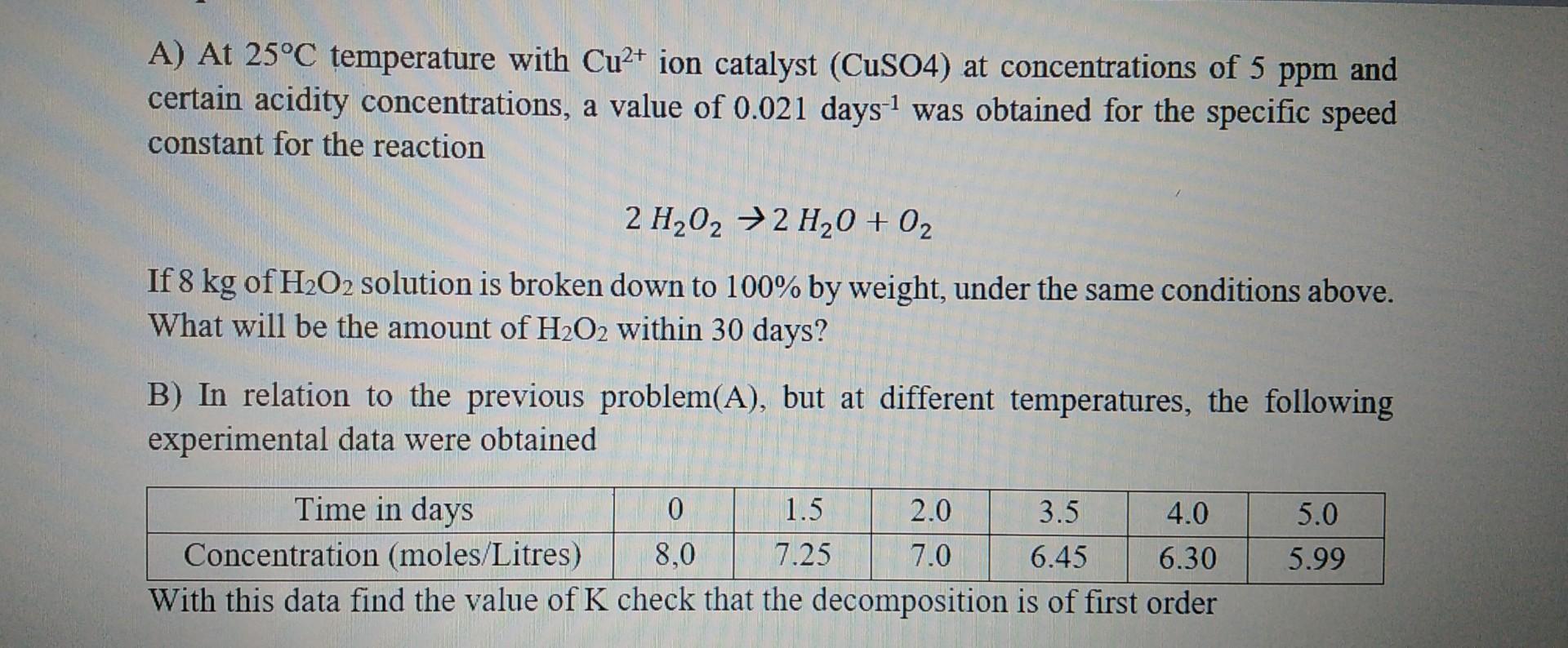
A) At 25C temperature with Cu2+ ion catalyst (CuSO4) at concentrations of 5ppm and certain acidity concentrations, a value of 0.021 days 1 was obtained for the specific speed constant for the reaction 2H2O22H2O+O2 If 8kg of H2O2 solution is broken down to 100% by weight, under the same conditions above. What will be the amount of H2O2 within 30 days? B) In relation to the previous problem(A), but at different temperatures, the following experimental data were obtained With this data find the value of K check that the decomposition is of first order
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


