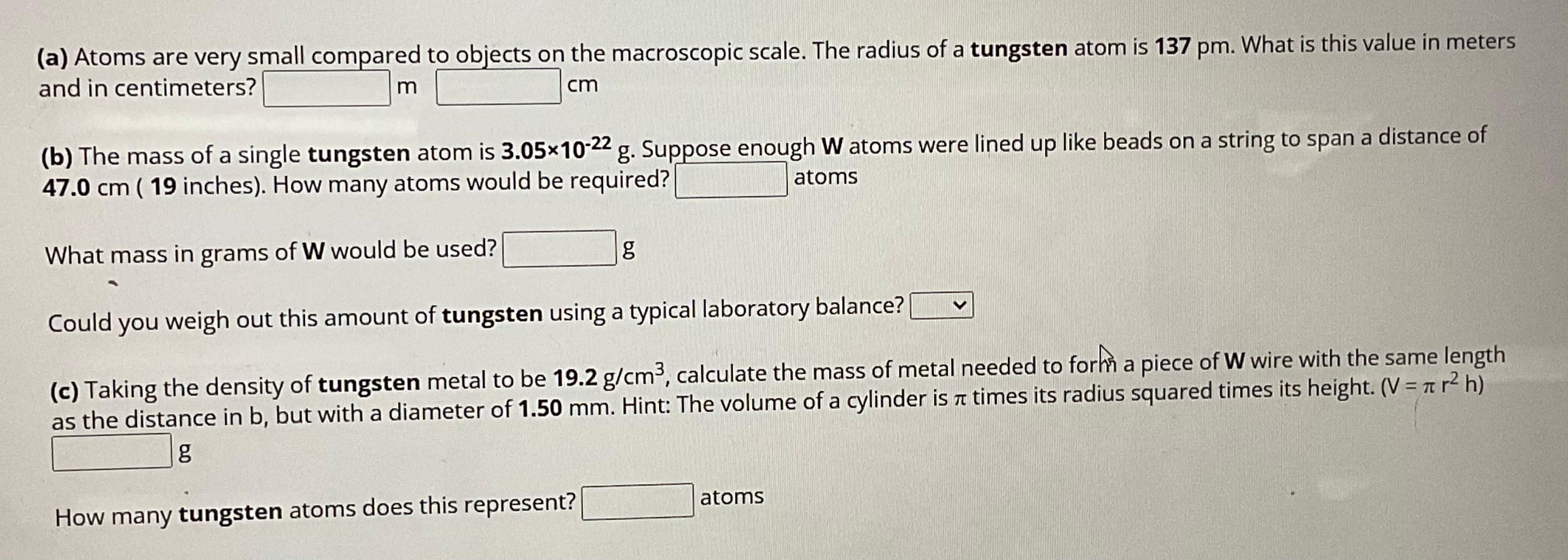
Question: ( a ) Atoms are very small compared to objects on the macroscopic scale. The radius of a tungsten atom is 1 3 7 p
a Atoms are very small compared to objects on the macroscopic scale. The radius of a tungsten atom is What is this value in meters and in centimeters?
b The mass of a single tungsten atom is Suppose enough atoms were lined up like beads on a string to span a distance of inches How many atoms would be required? atoms
What mass in grams of would be used?
g
Could you weigh out this amount of tungsten using a typical laboratory balance?
c Taking the density of tungsten metal to be calculate the mass of metal needed to forhn a piece of W wire with the same length as the distance in but with a diameter of Hint: The volume of a cylinder is times its radius squared times its height. Vg
How many tungsten atoms does this represent? atoms
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


