Question: A, b and c please rmat Arrange Tools Slide Show Window Help ns Slide Show Review View be X X A- A- FBBS2 Reagent Workshop
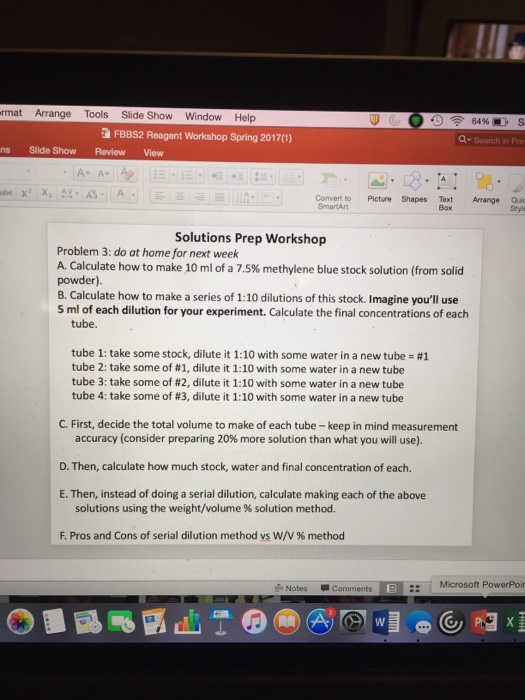
rmat Arrange Tools Slide Show Window Help ns Slide Show Review View be X X A- A- FBBS2 Reagent Workshop Spring 2017(1) AS Convert to SmartArt Solutions Prep Workshop Picture Shapes tube 1: take some stock, dilute it 1:10 with some water in a new tube = #1 tube 2: take some of #1, dilute it 1:10 with some water in a new tube tube 3: take some of #2, dilute it 1:10 with some water in a new tube tube 4: take some of # 3, dilute it 1:10 with some water in a new tube Problem 3: do at home for next week A. Calculate how to make 10 ml of a 7.5% methylene blue stock solution (from solid powder). B. Calculate how to make a series of 1:10 dilutions of this stock. Imagine you'll use 5 ml of each dilution for your experiment. Calculate the final concentrations of each tube. Notes D. Then, calculate how much stock, water and final concentration of each. E. Then, instead of doing a serial dilution, calculate making each of the above solutions using the weight/volume % solution method. F. Pros and Cons of serial dilution method vs W/V % method C. First, decide the total volume to make of each tube - keep in mind measurement accuracy (consider preparing 20% more solution than what you will use). Comments Text W 64 % S Q Search in Prom www Arrange Quic Style Microsoft PowerPoin
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
A 75 of methylene blue means 75 gm methylene blue in 100 ml of solution Required volume 10ml Now for ... View full answer

Get step-by-step solutions from verified subject matter experts


