Question: A + B B + B , - r A = k C A C B The following reaction is to be operated under the
The following reaction is to be operated under the conditions of CA molm and CB molm
To minimize the overall volume of a reactor to obtain the given conversion rate, show which flow reactor or combination of reactors is optimal. It is not necessary to calculate the size of a reactor.
conversion
conversion
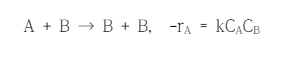
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


