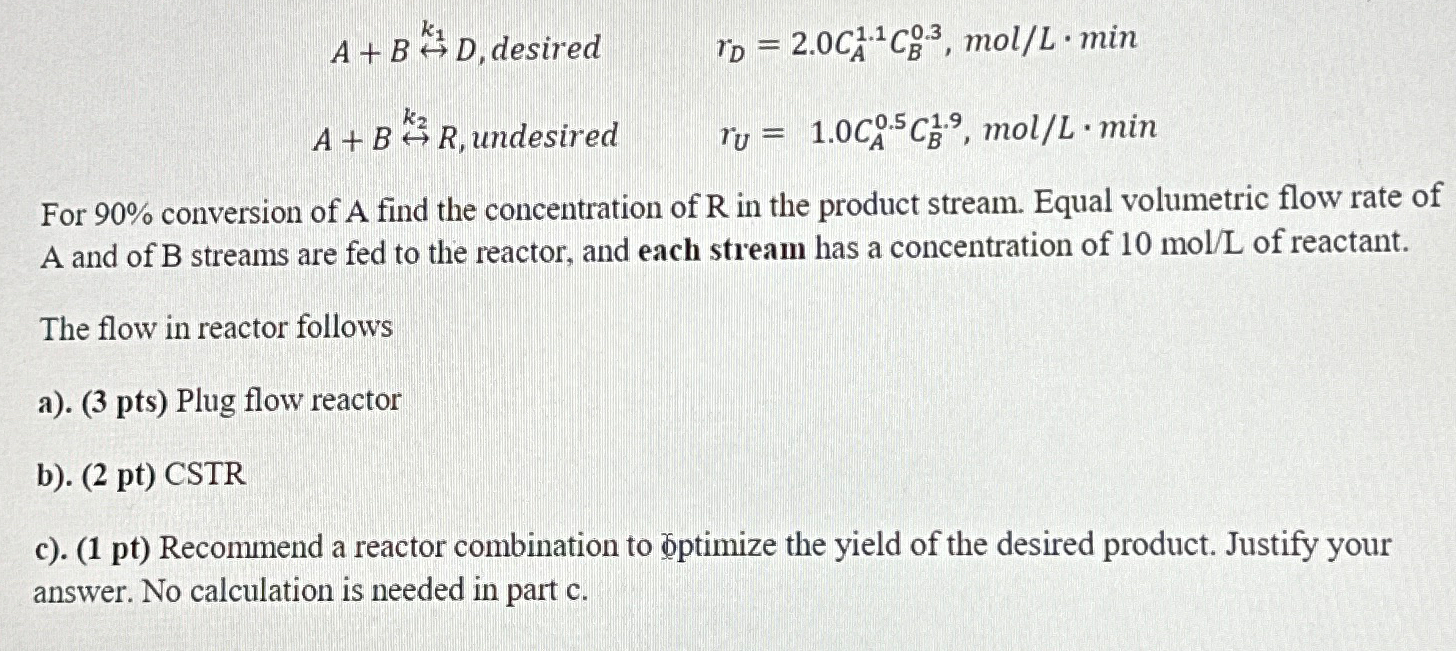
Question: A + B h a r r k 1 D , desired r D = 2 . 0 C A 1 . 1 C B
desired
undesired
For conversion of A find the concentration of in the product stream. Equal volumetric flow rate of A and of B streams are fed to the reactor, and each stream has a concentration of of reactant.
The flow in reactor follows
a pts Plug flow reactor
b pt CSTR
c pt Recommend a reactor combination to answer. No calculation is needed in part c
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


