Question: A Beer-Lambert law can be applied to a mixture when there are two or more solutes in the solution: Atet = A1 + A2 +
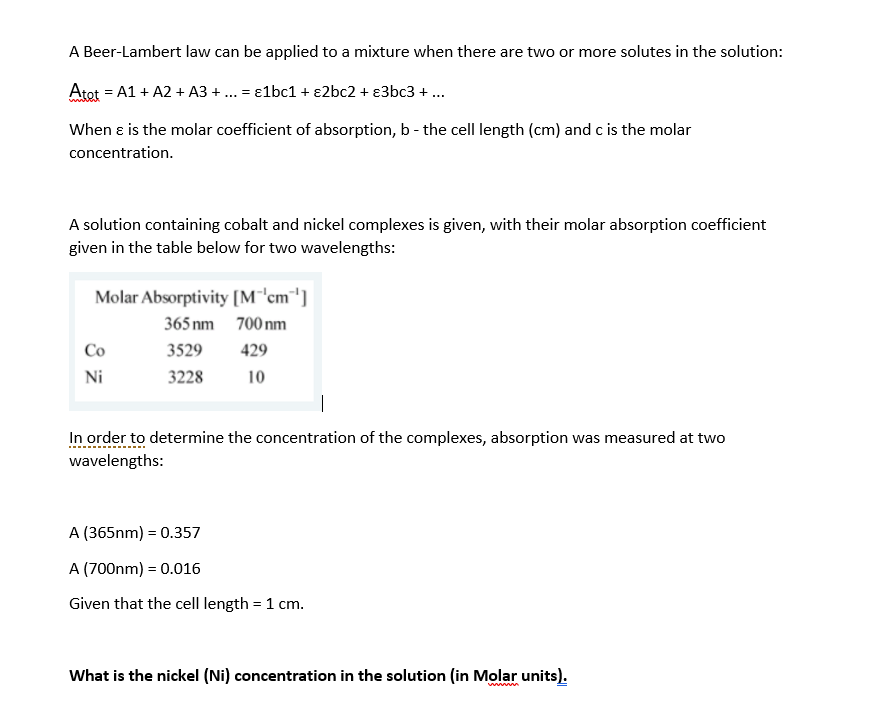
A Beer-Lambert law can be applied to a mixture when there are two or more solutes in the solution: Atet = A1 + A2 + A3 + ... = 1bc1 + 2bc2 + 3bc3 + ... When e is the molar coefficient of absorption, b- the cell length (cm) and c is the molar concentration. A solution containing cobalt and nickel complexes is given, with their molar absorption coefficient given in the table below for two wavelengths: Molar Absorptivity [M"'cm') 365 nm 700 nm Co 3529 429 Ni 3228 10 the complexes, absorption was measured at two In order to determine the concentration wavelengths: A (365nm) = 0.357 A (700nm) = 0.016 Given that the cell length = 1 cm. What is the nickel (Ni) concentration in the solution (in Molar units)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


