Question: (a) Between 0C and 100C determine the equilibrium conversion for the elementary aqueous reaction Here, assume that AH rxn is not a SAG298 = -14
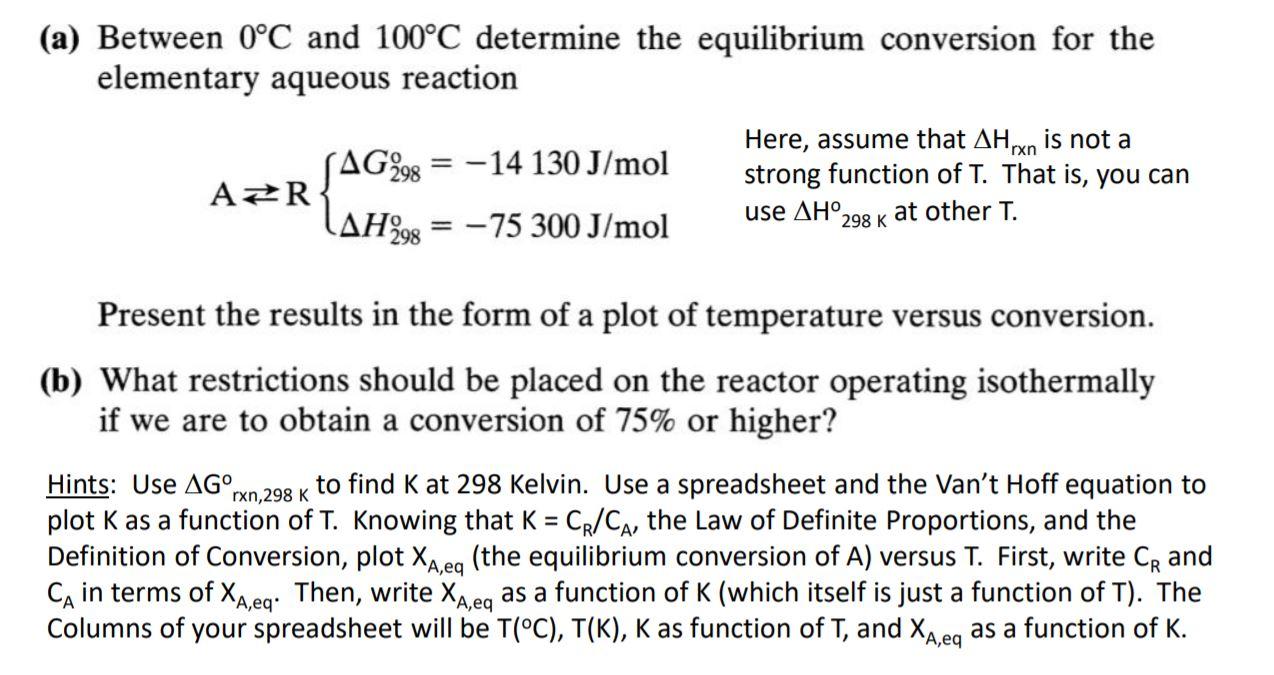
(a) Between 0C and 100C determine the equilibrium conversion for the elementary aqueous reaction Here, assume that AH rxn is not a SAG298 = -14 130 J/mol AZR strong function of T. That is, you can at other T. LAH298 = -75 300 J/mol = use . 298 K Present the results in the form of a plot of temperature versus conversion. (b) What restrictions should be placed on the reactor operating isothermally if we are to obtain a conversion of 75% or higher? rxn,298 K Hints: Use AG to find K at 298 Kelvin. Use a spreadsheet and the Van't Hoff equation to plot K as a function of T. Knowing that K = CR/CA, the Law of Definite Proportions, and the Definition of Conversion, plot XA,eq (the equilibrium conversion of A) versus T. First, write CR and Ca in terms of Xx,eg. Then, write XA,eq as a function of K (which itself is just a function of T). The Columns of your spreadsheet will be T(C), T(K), K as function of T, and Xaneq as a function of K. A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


