Question: A binary mixture consists of ethanol (1) and n-hexane (2). If the liquid equilibrium mixture composition x1 is 0.528 mole fraction of ethanol at 334K,
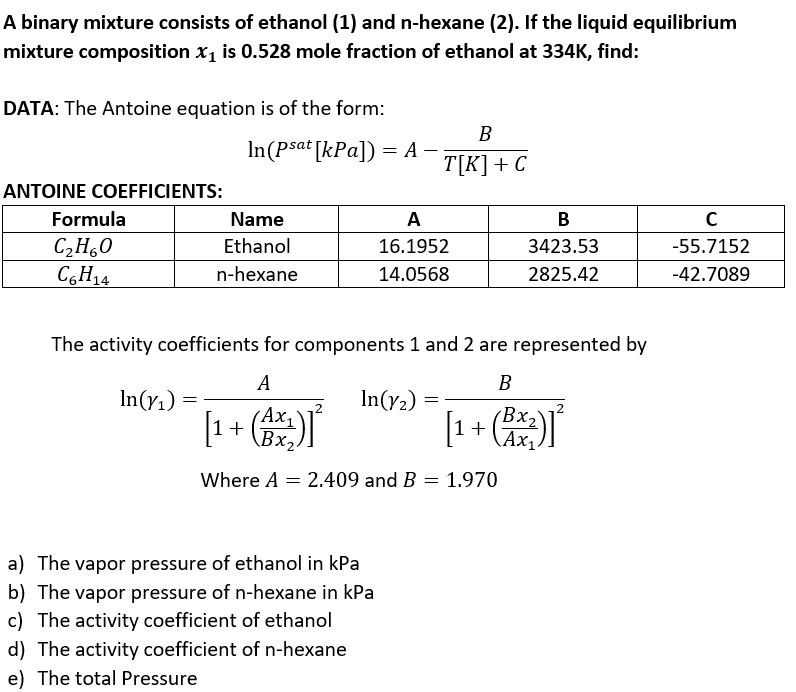
A binary mixture consists of ethanol (1) and n-hexane (2). If the liquid equilibrium mixture composition x1 is 0.528 mole fraction of ethanol at 334K, find: DATA: The Antoine equation is of the form: ln(Psat[kPa])=AT[K]+CB ANTOINE COEFFICIENTS: The activity coefficients for components 1 and 2 are represented by ln(1)=[1+(Bx2Ax1)]2Aln(2)=[1+(Ax1Bx2)]2BWhereA=2.409andB=1.970 a) The vapor pressure of ethanol in kPa b) The vapor pressure of n-hexane in kPa c) The activity coefficient of ethanol d) The activity coefficient of n-hexane e) The total Pressure
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


